
Oregon Health Authority
Pharmacy Benefit Managers
Poor Accountability and
Transparency Harm
Medicaid Patients and
Independent Pharmacies
August 2023
Report 2023-25
November 8, 2023 - An update to this
report can be found in footnote twenty
on page 16.

Billions of dollars are spent on Medicaid. It is important the state take steps to ensure the program is run
efficiently and effectively to better serve people in Oregon. The current structure lacks transparency and is too
complex to efficiently measure value. The State should change the current model and enact legislation that
focuses on patient protections, pharmacy protections, and increasing transparency in the prescription drug supply
chain. Making these changes will help ensure the Medicaid program is getting good value for pharmacy benefits,
people have access to the same medications, and Oregonians have access to community pharmacies.
Why this audit is important
•
Prescription drugs reduce the need
for medical services and improve and
extend life. While efforts to lower
drug prices have targeted
manufacturers, there is growing
interest in reviewing the influence of
pharmacy benefit managers (PBMs).
•
The largest PBMs in the U.S. control
80% of the market share and are
vertically integrated with the largest
health insurance companies and
pharmacies. Vertical integration
poses risks to drug affordability and
decreases access to medications.
•
CCO patients do not have access to
the same medications under the
current model. Moving to another
CCO could result in patients needing
to go through the burdensome prior
authorization process.
•
Adopting leading practices will
improve pharmacy access, improve
transparency in the prescription drug
process, and potentially save
taxpayer dollars.
What we found
1. The current structure of Medicaid PBMs is too complex for the State
of Oregon to efficiently measure value
. The prescription drug process
in Medicaid involves multiple entities including sixteen CCOs
(Coordinated Care Organizations), six PBMs, hundreds of
pharmacies,
multiple drug manufacturers, wholesalers, pharmacy administrative
organizations, OHA, and the Department of Consumer and Business
Services, among others. (pg.
6)
2. Oregon’s regulation of PBMs is limited and fragmented. Other states
have meaningful legislation targeted at patient protections,
pharmacy protections, and transparency. PBM reforms are bipartisan
policy efforts to limit unfair practices, which can hurt community
pharmacies and limit access for people. Other states are also
adopting different PBM models for Medicaid, making it easier for
governments to provide effective oversight. (pg.
14)
3. Pharmacy reimbursements vary significantly depending on the
drugs,
pharmacy type, and PBM. Pharmacies often lose money when filling
certain prescriptions. We found that national chains, some of which
are owned by PBMs or PBM parent companies, were reimbursed
twice the amount independent pharmacies were for selected drugs.
(pg.
20)
4. OHA does not ensure sufficient transparency and compliance from
PBMs. While OHA has improved CCO contract language, more needs
to be done to ensure high-
risk areas are monitored appropriately and
contract provisions are comprehensive. (pg. 28)
rebate
Audit Highlights
Oregon Health Authority, Pharmacy Benefit Managers
Poor Accountability and Transparency Harm Medicaid Patients and
Independent Pharmacies
What we recommend
We made 2 recommendations to OHA and 7 to the Legislature. OHA agreed with all of our recommendations. The
response can be found at the end of the report.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 1
Introduction
Prescription drugs reduce the need for medical services and improve and extend life. The ever-
increasing cost of prescription drugs in the U.S. is a topic of national interest. The high price of
medications can reduce consumers’ access and contributes to higher spending, straining both state and
federal budgets. From 2009 to 2018, national spending on prescription drugs in the Medicaid program
increased from $18 billion to $32 billion.
The prescription drug system in the U.S. is complex, involving many entities. While some of the efforts
to decrease drug prices in recent years have targeted drug manufacturers, there is growing public
interest in assessing the role, value of, and significant power and influence held by third-party
organizations known as pharmacy benefit managers.
Pharmacy benefit managers are influential within the U.S. health care
system
The supply of prescription drugs starts with drug manufacturers, who develop and manufacture
medications. Some of the largest drug manufacturers in the world include Johnson & Johnson, Eli Lilly,
Pfizer, and AbbVie. Drug manufacturers then sell medications to wholesale distributors, who resell
those drugs directly to pharmacies or collective groups who pool resources and negotiate on their
behalf.
1
The final step in the supply chain happens when pharmacies fill and dispense medications to
consumers.
Involved in many of these processes are third-party companies known as pharmacy benefit managers
(PBMs). Today’s PBMs have emerged as powerful intermediaries between insurers, manufacturers,
pharmacies, and governments, though historically they were created as simple claims processing
administrators. Within this paradigm, the three largest PBM’s control 80% of the U.S. prescription
market as seen in Figure 1.
Private sector insurers first started covering high volumes of prescription drugs for health plans in the
1960s. When this happened, insurers had challenges in managing the overall increase in claims. Early
PBMs were initially created to ease the administrative burden of insurers. Over time, the role of PBMs
has expanded significantly.
In the decades after their initial creation, PBMs leveraged new technologies as they emerged to
streamline and simplify administrative processes, like creating real-time electronic claims processing
and efficient pharmacy communication methods. PBMs also began to offer new services over time to
insurers, like mail order, pharmacy networks, and clinical consulting, among others. In the 90’s, drug
manufacturers began acquiring PBMs, which created conflict of interest concerns. The Federal Trade
Commission ordered the manufacturers to divest the businesses and started a trend of mergers and
acquisitions within the PBM industry. Some of the current responsibilities of PBMs include:
• Processing and paying prescription drug claims;
1
The term collective groups is used in this report to refer to the combination of pharmacy services administration organizations
and group purchasing organizations. A definition of these entities, as well as a full glossary of terms, can be found in Appendix A.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 2
• Creating a preferred drug list, which is a list of prescription drugs a health plan will
cover for its beneficiaries;
• Negotiating prices and rebates with drug manufacturers;
• Contracting with pharmacies and collective groups who negotiate on behalf of
pharmacies to create pharmacy networks; and
• Drug utilization reviews, which analyze the prescribing, dispensing, and use of
medications.
Certain PBM practices create risks for private insurers and federal and state health
programs
PBMs have merged with other entities to remain competitive and to increase their revenue streams.
Today, the largest PBMs are vertically integrated with the largest health insurance companies and retail
and mail order pharmacies.
2
Some of these large PBMs are contracted to provide pharmacy benefits for
many of Oregon’s coordinated care organizations in Medicaid.
Figure 1: Three companies make up 80% of the 2020 market share of claims for PBMs
Source: Health Industries Research Companies
PBMs have considerable influence on which drugs are covered by insurers and can require consumers
to get certain prescriptions filled at a specialty or mail order pharmacy, which the PBM may own. CVS
Health, a vertically integrated system, noted in recent financial statements the rebates they receive
from drug manufacturers often depend on whether the PBM places their drugs on a health plan’s
preferred drug list. Vertical integration in the pharmaceutical system poses risks of decreased
consumer access to medications and affordability to everyone, not just those receiving Medicaid
benefits.
2
Vertical integration in health care refers to the mergers and acquisitions of companies that offer diversified services and/or
products across a continuum of health care services.
CVS Caremark
34%
Express Scripts
25%
Humana 8%
Prime Therapeutics 6%
MedImpact 4%
All others 3%
OptumRx
21%
The United States is the only developed country that uses PBMs for its public health programs.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 3
Figure 2: A hypothetical example of a patient who receives care from vertically integrated entities
Source: Auditor created based on CVS and subsidiary information
PBMs are involved in many aspects of the prescription drug supply chain, and because of that, there is
a risk that reported cost savings may not be accurately reflected. For example, a manufacturer lists the
price of a drug at $10. The PBM tells the insurer they can negotiate a discount on the drug, but then
the list price goes up to $25. The PBM negotiates with the manufacturer to get the price back down to
$10 and can then report to the insurer that they saved $15 on that prescription, when the cost did not
actually change from $10. Depending on the contract a PBM has with an insurer, they may get to keep
a portion of the rebates, or discounts, received from drug manufacturers.
The deals PBMs negotiate with insurers, manufacturers, pharmacies, and other entities are often
considered trade secret information and do not have to be shared. This opaque system makes it
impossible to understand the actual costs of prescription drugs and has garnered attention at multiple
levels of government.
In 2022, the Federal Trade Commission announced it would launch an inquiry into PBMs, requiring them
to provide information and records regarding business practices owing to the lack of transparency and
size of the largest PBMs. The inquiry will target high-risk PBM practices, such as:
• Fees and clawbacks charged to unaffiliated pharmacies;
3
3
Practice of charging co-payments to consumers for certain prescription drugs that exceed the cost of medicines, with the
difference required to be returned to the PBM by the pharmacy.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 4
• Methods to steer patients toward PBM-owned pharmacies;
• Potentially unfair audits of independent pharmacies;
• Complicated and opaque methods used to determine pharmacy reimbursements;
• The prevalence of prior authorizations and other administrative restrictions;
• The use of specialty drug lists and related specialty drug policies; and
• The impact of rebates and fees provided by drug manufacturers on preferred drug
list design and the costs of prescription drugs to payers and patients.
Spread pricing is another high-risk area. Spread pricing has been cited as costing governments,
pharmacies, and patients more money for the delivery of prescription drugs. Spread pricing occurs
when a PBM keeps the difference between what is charged to the health plan and what is reimbursed
to a pharmacy. For example, an insurer agrees to pay a PBM $100 for a prescription, but the PBM’s
contract with the pharmacy states it will reimburse the pharmacy $75. If the PBM keeps the $25
difference, this is considered spread pricing. Health plans often do not know the amounts reimbursed
to pharmacies, as PBMs would consider that information proprietary. At least 11 states have banned
spread pricing in their Medicaid programs. In Oregon, CCOs and their PBMs are permitted to split the
spread, depending on their contracts.
Figure 3: Spread pricing happens when PBMs keep the difference between what is paid to health plans and
pharmacies
Some contracts with PBMs state the difference will be split between them. In the example above, both
the insurer and PBM would each keep $12.50 if the difference were split evenly. Oregon’s Medicaid
program allows PBMs to operate under either a pass-through contract or a model where the PBM and
CCO each receive a portion of the spread, known as a pay-for-performance contract. While spread
“Although many people have never heard of pharmacy benefit managers, these
powerful middlemen have enormous influence over the U.S. prescription drug
system.”
Lina M. Khan, Federal Trade Commission Chair

Oregon Secretary of State | Report 2023-25 | August 2023 | page 5
pricing poses risks, there are other transactions outside of the claims payment process possibly
affecting costs at the pharmacy level and PBM revenues. There are additional fees PBMs can receive
from insurers, pharmacies, and drug manufacturers that are considered proprietary information, which
makes it even more challenging for Medicaid programs to truly understand the actual cost of pharmacy
benefits.
Medicaid, the largest and most complex government program in
Oregon, uses PBMs for most pharmacy benefits
Across the nation, Medicaid programs and public employee health plans are evaluating their
relationships with PBMs due to concerns about the complexity of the process, transparency, and rising
costs. Medicaid is a government program providing health care coverage to low‐income adults,
children, pregnant people, the elderly, and people with disabilities. It is financed through federal and
state funding and is administered by each state.
Figure 4: Oregon spends more on Medicaid than any other program
Source: Auditor-created based on the State of Oregon 2020 Financial Condition Report
In 2020, Oregon spent more on the Medicaid program than it did on any other area, including education,
transportation, and public safety combined.
4
As of January 2023, about 1.4 million people in Oregon
receive Medicaid benefits. In 27 counties in Oregon, more than one-third of the population receives
Medicaid benefits.
The federal government estimates national Medicaid expenditures will increase to over $1 trillion by
2028; state officials estimate the cost of health care in Oregon will grow faster than the state’s
4
State of Oregon 2020 Financial Condition Report
Medicaid
32%
Education
19%
Other
15%
Unemployment
13%
Human services
10%
Transportation
5%
Public safety
5%

Oregon Secretary of State | Report 2023-25 | August 2023 | page 6
economy. Due to the size and dollar amounts flowing into this program, it is important the state take
steps to ensure the program is run efficiently and effectively to better serve people in Oregon.
Figure 5: In most Oregon counties, one-in-three people received Medicaid benefits in 2022
Source: OHSU’s Office of Rural Health
The Oregon Health Authority administers Medicaid in Oregon
Oregon’s Medicaid program, also known as the Oregon Health Plan, is administered by the Oregon
Health Authority (OHA).
5
OHA’s 2021-23 biennial budget is over $32 billion with 5,100 full-time
employees across seven divisions. OHA works closely with other state and local agencies, as well as
Tribal governments, to provide services and health care to people in Oregon.
Medicaid is the largest program under OHA and accounts for 68% of the agency’s budget. OHA’s Health
Systems Division oversees the Medicaid program and sets guidelines regarding eligibility and services in
accordance with federal requirements.
The Oregon Department of Human Services also oversees some Medicaid-funded programs that
provide care to clients in their own homes or communities. These programs serve eligible, low-income
individuals. Although the department operates some pieces of Medicaid, ultimate state responsibility
for the program falls to OHA.
Six PBMs provide pharmacy benefits for all 16 of Oregon’s Coordinated Care
Organizations
Oregon uses both fee-for-service (FFS) and coordinated care models to deliver services. The FFS
model is the more straightforward of the two: a Medicaid client visits a health care provider, the
5
Oregon Health Plan coverage also includes the Children’s Health Insurance Program, Reproductive Health Equity Act, Cover All
Kids, and the Cover All People programs.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 7
provider bills OHA directly for approved services, and OHA pays the provider. About 10% of Medicaid
clients in Oregon are FFS.
6
Figure 6: Coordinated Care is a more indirect payment system than FFS
The coordinated care model involves coordinated care organizations, or CCOs. A CCO is a network of all
types of health care providers (physical, behavioral, and dental care providers) who work together in
their local communities to serve people who receive coverage under Medicaid. CCOs focus on
prevention and helping people manage chronic conditions. Oregon currently has 16 CCOs providing
coverage across the state.
Figure 7 shows a simplified version of the complex relationships PBMs, CCOs, OHA, and other entities
have in the Medicaid prescription drug system. A more complete chart that accurately depicts these
complex relationships can be found in Appendix B.
6
Mental health drugs are carved out from coordinated care benefits, even if the person is enrolled in a CCO. This 10% only
includes people not enrolled in a CCO.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 8
Figure 7: Medicaid PBMs operate in a complex environment involving many different entities
Source: Auditor-created, simplified model of Oregon’s PBM structure based on information from OHA and the Congressional
Budget Office. See also Appendix B
for a comprehensive flowchart of Oregon’s pharmaceutical supply chain.
OHA pays CCOs predetermined rates, known as capitated payments, every month for each Medicaid
client for covered services. CCOs receive these payments no matter how many or how few services a
Medicaid client uses in a month. In 2023, the average capitated payment is $507.90, but rates vary
among CCOs, age ranges, and other factors.
CCOs pay providers and subcontractors, like PBMs, for services rendered and send encounter data to
OHA. This data shows the services provided to clients and helps set future rates for capitated
payments. Medicaid patients do not have out-of-pocket costs for prescription drugs, even if they have
additional health insurance, which is always billed first. Figure 8 lists the 16 CCOs, their PBM
subcontractors, and the number of people enrolled as of January 2023.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 9
Figure 8: CCOs currently contract with six entities who provide pharmacy benefits
CCO PBM(s)
7
January 2023 enrollment
Advanced Health MedImpact 27,204
AllCare MedImpact 61,884
Cascade Health Alliance MedImpact 25,520
Columbia Pacific OptumRx 35,344
Eastern Oregon Navitus (Oregon Prescription Drug
Program)
71,475
Health Share Multiple
8
427,339
InterCommunity Health Network OptumRx 80,126
Jackson Care Connect OptumRx 62,681
PacificSource- Central CVS Caremark 73,117
PacificSource- Columbia Gorge CVS Caremark 16,841
PacificSource- Lane CVS Caremark 89,098
PacificSource- Marion/Polk CVS Caremark 140,073
Trillium Community Health-
Southwest
Envolve Pharmacy Solutions 35,874
Trillium Community Health- Tri-
County
Envolve Pharmacy Solutions 38,381
Umpqua Health Alliance MedImpact 36,797
Yamhill Community Care Providence 34,778
FFS N/A 129,230
Total 1,385,762
Source: OHA PBM and Medicaid enrollment information
Pharmacy benefits continue to be a large spending category for CCOs
OHA estimates about 14% of each monthly capitated payment sent to CCOs is for prescription drugs. It
is important to note this estimate does not include drugs given in a hospital setting or drugs
administered by physicians. Mental health drugs are also excluded, as they are paid under the FFS
model, even if the person is enrolled in a CCO.
9
In 2021, about 11.8 million Medicaid prescriptions were
dispensed at pharmacies, and CCOs reported spending $767 million on prescription drug benefits, while
FFS drug expenditures were $208 million.
10
Generic drugs are the most prescribed medications, but specialty drugs make up a disproportionate
share of total expenditures. There is no single agreed-upon definition of a specialty drug between PBMs
and insurers. Definitions vary, but can include high-cost medications, drugs that require special
handling, availability limited to certain pharmacies, and drugs that treat rare diseases. Some generic
drugs can cost $2 or less, while some specialty drugs can cost over $100,000 per prescription. Generics
7
Some CCOs have changed PBMs in the past five years. Figure 7 reflects the CCO-PBM relationships as of January 2023.
8
Health Share has multiple subcontractors, each with their own pharmacy benefit relationship. These include OHSU, Providence,
Kaiser, CareOregon, OptumRx, and Legacy/Pacific Source.
9
Per OAR 410-141-3855, mental health drugs in Standard Therapeutic Classes 7 and 11 are carved out from coordinated care
benefits. Providers bill OHA directly for these medications.
10
The total amount paid does not reflect any rebates received that would reduce the total amount spent. FFS and CCO claims
are both subject to federally mandated rebates.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 10
are typically lower priced than brand name drugs, but the cost of generics has also been rising. Figure 9
demonstrates the disparate share specialty drugs have on total drug expenditures at one CCO.
Figure 9: Two thirds of Medicaid prescription drug spending is for specialty and brand name drugs, despite
comprising only 5% of claims
Source: OHA and PBM data
The amount of prescription drugs claims is increasing because the prevalence of chronic conditions has
increased with the aging of the U.S. population and because new therapies and generic drugs have
become more available. In the past several decades, the country has seen large growth in medications
treating common conditions, such as high blood pressure, high cholesterol, anxiety, and depression.
However, the number of generic drugs may not increase as much as brand and specialty drugs in the
future due to the likelihood of newer brand and biologic drugs and the current extensive use of
generics. Biologic drugs are complex and harder to manufacture than traditional prescription drugs.
Figure 10 details some of the most expensive drugs, in total, CCOs paid for in 2021.
0.4%
5%
90%
33%
33%
20%
0% 20% 40% 60% 80% 100%
Specialty drugs
Brand drugs
Generic drugs
% Claims % Spending
Specialty drugs make up less than 1% of prescriptions but are one-third of total prescription drug spending.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 11
Figure 10: CCOs spent more on Humira than any other drug at pharmacies in 2021
Brand name(s) Conditions treated
11
Claims Amount
Humira Crohn’s disease, ulcerative
colitis, plaque psoriasis
7,991 $53 million
Biktarvy HIV 8,178 $29 million
Mavyret Hepatitis C 2,072 $27 million
Lantus, Basaglar
Kwickpen, Toujeo
Diabetes 71,038 $26 million
Trikafta Cystic fibrosis 912 $22 million
Stelara Crohn’s disease, ulcerative
colitis, plaque psoriasis
947 $20 million
Trulicity Type 2 diabetes 21,249 $18 million
Enbrel Plaque psoriasis, rheumatoid,
and psoriatic arthritis
2,626 $15 million
Eliquis Deep vein thrombosis 27,757 $14 million
Humalog Type 2 diabetes 42,706 $13 million
Source: OHA, the Food and Drug Administration, and Drugs.com
The average cost per prescription for more expensive drugs in Figure 10 ranges from $300 to $24,000.
Conversely, the most dispensed drugs — like albuterol sulfate, omeprazole, and ibuprofen — have an
average cost per prescription of $3 to $35.
Understanding the true cost of prescription drugs is difficult. Costs are often obscured by
nondisclosure agreements throughout the distribution chain. For example, wholesale acquisition cost is
the drug manufacturer’s list price to wholesalers; however, this is typically not the actual price paid.
The average wholesale price is the published list price for drugs sold by wholesalers to retail pharmacies
and is used as a starting point for negotiations.
Drug cost inputs are not always available and determining actual prices paid is difficult without access
to confidential information. Many negotiated drug prices are proprietary and known only to the parties
involved in the transaction. Drug manufacturers do not provide public information on how they set the
list price and have often not been required to explain changes in a drug’s list price.
12
PBMs add an
additional layer of complexity, as the prices paid to drug manufacturers and reimbursements to
pharmacies are also considered proprietary information. States are required to invoice manufacturers
for rebates on covered drugs for Medicaid. OHA has full visibility of the net cost of drugs under FFS, but
not in coordinated care. While increased transparency in prescription drug prices may not directly
lower costs, it will help policymakers better understand which prescription drugs and which portions of
the supply chain are cost drivers.
Oregon has placed an emphasis on drug manufacturers, but PBMs have received less
scrutiny
In 2018, the Oregon Legislature enacted the Prescription Drug Price Transparency Act. Housed in the
Department of Consumer and Business Services (DCBS), the act established the Task Force on Fair
11
Other conditions may be treated with these medications.
12
House Bill 2658 requires drug manufacturers to report to DCBS specific information on price increases for certain medications.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 12
Pricing of Prescription Drugs. The task force was directed to create a strategy for understanding the
pieces of the pharmaceutical supply chain and make recommendations to the Legislature to bring more
transparency. The task force made 15 recommendations, one of which focused on PBMs.
13
As of the
time of this report, only one of the recommendations has been fully implemented, one is partial, and 13
have not been implemented, which includes the PBM-focused recommendation.
After the recommendations were made, the task force was disbanded, and the Prescription Drug Price
Transparency Program took over at DCBS. The program compiles specific cost and price information
from manufacturers and health insurers and issues yearly reports on their findings.
14
However, most
efforts are focused on drug manufacturers.
Oregon is one of a handful of states in the country with a Prescription Drug Affordability Board. The
board was established in 2021 to protect stakeholders within Oregon’s health care system from the
high costs of prescription drugs. The board studies the prescription drug distribution and payment
system in Oregon and looks at policies adopted in other states and countries with the goal of lowering
the list price of prescription drugs for people in Oregon. The board releases two yearly reports detailing
its findings and recommendations.
15
One report is targeted to OHA and the Legislature, and the other is
an informational report on the generic drug marketplace.
DCBS is the regulator of the insurance industry in Oregon and is
charged with registering PBMs. Starting in 2013, PBMs must be
registered to conduct business in the state and renew annually.
While DCBS can also investigate complaints lodged against
PBMs for several reasons, staff noted complaints in the last few
years have been minimal and there have not been any
significant investigations aimed at PBM misconduct to date.
DCBS reported receiving 123 PBM complaints from 2015 – 2022
and three so far in 2023. Most of these complaints were closed
with no action determined to be needed, according to DCBS’s interpretation of Oregon or federal
statutes. Pharmacists we spoke to say the complaint process is onerous and ineffective; they have
stopped lodging complaints unless they are particularly egregious.
Current statutes define PBMs as organizations contracting with pharmacies on behalf of an insurer
offering a health benefit plan, a third-party administrator, or the Oregon Prescription Drug Program.
16
DCBS does not consider Medicaid PBMs to fall under the current definition; therefore, they are not
subject to most statutory requirements and go unregulated by DCBS. The Legislature should add
Medicaid PBMs to the definition in ORS 735.530.
13
Task Force on Fair Pricing of Prescription Drugs report
14
DCBS Prescription Drug Price Transparency reports
15
DCBS Prescription Drug Affordability Board Reports
16
ORS 735.530
Examples of PBM complaints DCBS
can investigate
• Maximum allowable cost violations
• Pharmacy auditing violations
• Fraud
• Patient steering
• Anti-gag clause violations

Oregon Secretary of State | Report 2023-25 | August 2023 | page 13
Prescription drugs are important for patients’ health and wellbeing, warranting
government regulation
The Federal Trade Commission enforces non-criminal antitrust laws in the U.S. to prevent and eliminate
anticompetitive business practices, including monopolies.
17
However, there are certain industries where
monopolies are allowed and are regulated for the public good, like utilities. Utility companies hold
natural monopolies over certain service areas. These natural monopolies provide greater efficiency and
economies of scale. To compensate for this, the government heavily regulates them to protect
consumers. Government agencies currently regulate utility companies for prices charged to customers,
budgetary processes, construction of new facilities, services offered, and energy efficiency programs.
These monopolies provide critical services that heat homes, water crops, and make economy
sustaining industry possible.
Utilities are essential to people’s wellbeing and a similar argument can be made for prescription drugs.
Patients need critical or lifesaving prescription drugs to treat high blood pressure, heart disease,
asthma, diabetes, mental illness, among many other conditions. Without these prescriptions, patients
would have a lower quality of life and possibly live shorter lives. Because a handful of PBMs control
most of the market and vertical integration has become more prevalent, there is a possibility the
industry is at risk of anti-competitive practices. Given the necessity for medications and their overall
importance to the public good, there is a need for reasonable government regulation.
“This unfair squeeze by PBMs on independent pharmacies in
Oregon and throughout the country poses a direct threat to
these community mainstays’ ability to stay open for their
patients who count on them for quality local service.”
-
Ron Wyden, United States Senator (Oregon)
Source: Image courtesy of www.wyden.senate.gov
As part of our strategic effort to provide real-time auditing services, we provided timely information to
Oregon’s Legislature in September 2022 and February 2023, as seen in Appendix C. Real-time auditing
focuses on evaluating front-end strategic planning, service delivery processes, controls, and
performance measurement frameworks before or at the onset of significant program or public policy
implementations by state agencies.
17
Investopedia What Is a Monopoly? Types, Regulations, and Impact on Markets A monopoly is a market structure where a single
seller or producer assumes a dominant position in an industry or a sector. Monopolies are discouraged in free-market economies
as they stifle competition and limit substitutes for consumers.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 14
Audit Results
Prescription drug prices have become a major concern across the U.S. In 2021, Oregon spent
approximately $767 million a year on retail prescriptions for Oregon Health Plan CCOs. While there have
been state and federal efforts to control drug prices, these efforts have primarily focused on drug
manufacturers while PBM risks, including a lack of transparency, have largely been overlooked. Other
states have passed meaningful legislation requiring more accountability from PBMs that have brought
more transparency to the prescription drug supply chain. To date, there has been some PBM reform
legislation proposed in Oregon, but more should be done.
This audit identified areas of concern including the current structure of Medicaid PBMs in Oregon and
OHA’s monitoring controls over CCOs and their contracted PBMs. The current structure lacks
transparency and is overly complex — as a result, it is difficult to determine the value provided to the
program and to people in Oregon. Transparency and accountability are obscured by nondisclosure
agreements and proprietary information. The current system does not support local community
pharmacies, which are a critical component of health care for all people in Oregon, not just those
receiving Medicaid benefits.
Oregon’s Legislature should follow leading practices in other states and create a universal preferred
drug list for Medicaid, require PBMs to act as fiduciaries, prioritize fair pharmacy reimbursements,
require PBMs to disclose cost information, and adopt a new PBM structure in Medicaid.
We also found PBM provisions in the CCO contracts have been strengthened, but OHA’s monitoring
controls are not sufficient to determine compliance and do not cover high-risk areas. Oregon has an
opportunity to regulate PBMs to increase the value they provide to the Medicaid program by adopting
leading practices to improve pharmacy access, improve transparency in the prescription drug process,
and potentially save taxpayer dollars.
Oregon’s Medicaid program cannot assess the public benefit of
hundreds of millions paid to pharmacy benefit managers
Regulation of PBMs in Oregon is limited and fragmented. DCBS monitors PBMs operating in the
commercial insurance space but not in Medicaid. Medicaid PBMs are subcontractors of CCOs, and OHA
does not have direct supervision over them. Statutory changes are needed in order to provide the
state with direct oversight of all PBMs.
Other states have passed laws increasing protections for patients and community pharmacies related
to PBMs. Some of these protections include uniform preferred drug lists, fair pharmacy
reimbursements, increased transparency, and changes to state Medicaid PBM models. We recommend
Oregon’s Legislature consider addressing all of these factors, to the benefit of the many Oregonians
who rely on prescription medications.
Oregon has been slow to address PBM reforms, falling behind many other states that
have passed significant PBM legislation
Policymakers across the country are taking different approaches in tackling rising prescription drug
costs. Other states have focused efforts on PBM reforms, while Oregon continues to focus primarily on

Oregon Secretary of State | Report 2023-25 | August 2023 | page 15
drug manufacturers. Oregon took some early steps to reduce prescription drug costs, but progress
since then has been minimal compared to other states
One early step Oregon took was the creation of the Oregon Prescription Drug Program (OPDP), which
is administered by OHA. OPDP was established in 2003. Currently, the program purchases prescription
drugs, reimburses pharmacy claims, creates a standard preferred drug list, and operates a prescription
discount card program.
18
One of OPDP’s goals is to ensure the most effective drugs are available at the
best prices to the insurers who use the program. OPDP eventually joined Washington State and formed
a consortium in 2006, expanding the purchasing power of the program. In 2022, the State of Nevada
joined the consortium and expansion to other states continues to be explored.
Currently, the program administers pharmacy benefits for 13 different institutions and programs across
three states, including the State Accident Insurance Fund, Oregon Health and Sciences University, and
the State Hospital and covers over 225,000 lives just within Oregon. CCOs can also choose OPDP to
administer pharmacy benefits; however, only one has chosen to do so.
In 2019, legislation was passed stating PBMs could not prohibit a network pharmacy from offering
delivery of prescription drugs to consumers. In the same bill, the Legislature prohibited PBMs from
restricting or penalizing a network pharmacy from informing a customer of the difference between
their out-of-pocket cost and the pharmacy's retail price for the drug. This was implemented in Oregon
after several other states had passed similar legislation. Many other states are currently making inroads
to dealing with spread pricing and PBM transparency issues as well. Although the Legislature has
demonstrated bipartisan interest in reforming PBMs, other issues have taken priority, including the
pandemic and several natural disasters.
The pharmaceutical industry also has strong lobbying power in the Oregon Legislature and has donated
more than $600,000 to various campaigns and funds over the past five years. The pharmaceutical
supply chain is very complex and can be difficult to understand. Since pricing and cost information can
be considered proprietary, policymakers do not have enough information available to enact meaningful
change for some issues.
There are three main areas other states have focused their PBM legislation on: patient protections,
pharmacy protections, and transparency. With the national spotlight currently focused on both drug
manufacturers and PBMs, Oregon’s Legislature made some progress during the 2023 session. Bills were
passed requiring PBMs to report some information to DCBS, pharmacy retroactive fees and payment
reductions were restricted, and recurring surveys of pharmacy dispensing costs were implemented.
While these are improvements, the Legislature should do more to protect patients and community
pharmacies.
18
ArrayRx is Oregon’s prescription discount card program, which has no income restrictions or membership fees, and is available
to anyone living in Oregon.
Oregon’s PBM legislation
In the last 10 years, 28 PBM reform bills have been introduced, but only seven have passed.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 16
Oregon’s PBM structure and current statute limit patient protections for prescriptions
A preferred drug list is a list of prescription drugs covered under a health plan. Health plans use
preferred drug lists to negotiate drug rebates and encourage providers to prescribe those medications
.
Uniform preferred drug lists support administrative simplicity for providers and consistent drug
coverage for patients.
Currently, Oregon has 16 different CCOs with multiple preferred drug lists. Having multiple preferred
drug lists in Medicaid creates challenges for patients, providers, and OHA. If people move to an area
with a different CCO, the prescription drug they previously took may or may not be on the new CCO’s
preferred drug list. If a person is prescribed a medication not on the list, extra steps need to be taken
before the individual can obtain their prescription.
For example, a person who lives in Coos Bay takes a blood pressure medication that works well for
them and was prescribed by their doctor. The person moved to Clackamas and now has a new CCO,
PBM, and preferred drug list. The original blood pressure medication might not be covered on the new
list. If this happens, the person might have to try new medications first, that may not work as well, or
the provider and patient will have to start the prior authorization process, which can be burdensome to
both patients and providers.
1920
PBMs will often move drugs on and off their preferred drug list depending on research and market
conditions. Frequent changes to covered drugs are burdensome, especially when changes are not
uniform across the state. In 2018, the Oregon Health Policy Board recommended alignment of the
preferred drug lists for FFS and CCOs to the Legislature but it was never implemented. Figure 11 shows
many other states have adopted this leading practice in their Medicaid programs. To bring consistency
to patients, Oregon’s Legislature should mandate a uniform preferred drug list for Medicaid. A standard
prior authorization process would be easier to implement under a uniform preferred drug list.
19
Prior authorization requires prescribers to receive pre-approval for prescribing a particular drug for that medication to qualify
for coverage under the terms of the pharmacy benefit plan. Prior authorization processes can differ among CCOs.
20
OAR 410-141-3850 requires CCOs to provide continued access to services when a Medicaid recipient moves from another CCO.
This rule does not apply to a Medicaid recipient who has a gap in coverage following disenrollment due to failure to respond to
mail from OHA that was sent to the recipient’s prior address. Under the existing model, there remains different access to
prescription drugs for Medicaid recipients depending based on where they live.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 17
Figure 11: Many states have adopted policies for uniform preferred drug lists for all or some drug classes in
Medicaid coordinated care
Source: Kaiser Family Foundation
Another patient protection other states have implemented is requiring PBMs to act as a fiduciary.
Fiduciaries are organizations acting on behalf of another person or entity, with a duty to preserve good
faith and trust, putting their client’s interests ahead of their own. Fiduciaries are legally and ethically
bound to act in the best interest of the people they serve.
Several states require all PBMs operating within the state, including commercial and Medicaid PBMs, to
sign contracts that include fiduciary clauses. According to these clauses, not only will the PBM act in
the best interest of the insurer they subcontract with, but they will also act in the best interest of the
beneficiaries of the insurer. This helps ensure the PBM will act in their self-interest only if the act does
not harm or reduce value to the program or the person. State Insurance Commissions are typically the
oversight body in states that have adopted this leading practice.
Pay-for-performance contracts create confusion and obstacles to transparency
CCOs may choose between contracting with OPDP or selecting a different PBM to provide services, as
they almost all have. CCOs might choose to use a different PBM, rather than OPDP, because they offer
other insurance plans outside of Medicaid that they already have a contracted PBM for. If CCOs choose
to use a different PBM, they can decide between two contract types: pass-through or pay-for-
performance. A pass-through contract states the PBM will pass through any negotiated manufacturer
States with fiduciary clauses
California, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Minnesota, Nevada, New Jersey, New York,
Oklahoma, South Dakota, and Vermont.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 18
savings, like discounts or rebates, to the CCO. Under this model, administrative fees for each claim paid
to the PBM by the CCO are typically higher. When CCOs choose a pay-for-performance contract, the
PBM passes through rebates and usually sets a much lower administrative fee; however, the PBM will
share a quarterly payout, based on performance, with the CCO. This payout is based on two key
factors: negotiating drug discounts from manufacturers worth more than the amount stipulated in the
contract and paying a smaller per-claim dispensing fee to pharmacies than the fee stipulated in the
contract.
Determining rebate pass-through is more complicated in a pay-for-performance model. Auditors
examined aggregate rebate data for both contract models. The pass-through contracts were clearer to
understand, while pay-for-performance aggregate rebate and discount totals were difficult to
determine. By requiring all PBM contracts to be pass-through, OHA could increase transparency for
dispensing fees and drug rebates and improve its own ability to review and monitor these contracts.
Low or unfair reimbursement rates have been attributed to a decline in local, sole
proprietor pharmacies, a critical component of the state’s health care system
Pharmacies are a critical element of health care and are important in promoting health outcomes, but
the number of independent, smaller pharmacies in many parts of Oregon has been dwindling. Closures
have been driven in part by low or unfair reimbursement rates, making it difficult for these pharmacies
to stay open and retain staff. Some PBMs pay pharmacies about a dollar more for dispensing
prescriptions in underserved areas, but this increase likely does not cover operating costs for the
pharmacy. Dispensing fees paid to pharmacies under FFS are much higher. Taking legislative action to
protect reimbursement rates is a critical step in keeping pharmacies open to the benefit of all
Oregonians, not just those on Medicaid.
“When people truly need help, they come to the
most accessible health care professional in their
community... we serve over 3,000 square miles as
the only local providers. People literally drive hours
to get our help; whether it’s antibiotics, pain
medications following surgery, flu or covid
vaccinations, diabetic medications, or professional
advice on complex medication situations. The
services we provide are real and valuable!”
-
John Murray, Rph, independent pharmacy
owner in Heppner, written legislative
testimony in support of House Bill 3013 in
2023
Pharmacists are ideally positioned in their respective communities to address gaps in care by
collaborating with other health care providers, which can help eliminate health disparities. A 2016 study
found as the availability of pharmacies in a given area increased, hospital readmissions for people in

Oregon Secretary of State | Report 2023-25 | August 2023 | page 19
Oregon 65 or older decreased.
21
In the U.S., it is estimated medication nonadherence is associated with
125,000 deaths, 33%-69% of medication-related hospitalizations, and $100 to $300 billion in health
care services annually.
22
Pharmacists can help improve medication adherence and management of
chronic diseases and potentially reduce costly hospital readmissions.
The number of local and independent pharmacies has been falling across the nation. Pharmacy closures
disproportionately affect Black and Latino communities. Closures have also negatively impacted
Oregon’s rural communities, which have higher barriers to accessing medications.
23
Sixteen counties in
Oregon are below the national average for community pharmacies per 10,000 people, as shown in
Figure 12.
Figure 12: Sixteen Oregon counties are below the national average for community pharmacies
Source: Auditor analysis of Portland State University Population Center data, pharmacy data from the Oregon Board of
Pharmacy, and the National Institute of Health
Although the map shows that many rural counties are in line with national averages, the ratio mapped
does not consider the geographic size of a county. Some rural counties are over twenty times larger
geographically than their urban counterparts while most have far fewer than a twentieth of the
population. This means rural counties with only one or two pharmacies serving a large geographic area
can have a pharmacy to resident ratio in line with national averages. But the nearest pharmacy to many
residents still may be hours away. Fewer pharmacies in any given area translate to longer drive times
and increased wait times at existing pharmacies. Mail order pharmacies can be helpful to those in
21
Journal article- Pharmacy density in rural and urban communities in the state of Oregon and the association with hospital
readmission rates
22
Medication adherence generally refers to patients taking their medications as prescribed and as long as prescribed.
23
Journal article- Independently Owned Pharmacy Closures in Rural America

Oregon Secretary of State | Report 2023-25 | August 2023 | page 20
remote areas, but do not provide the same in-person care as community pharmacies. Sometimes there
are issues with timely deliveries, which can be critical for people on certain medications.
Low or unfair reimbursement rates have been cited as a key factor for pharmacy closures. A national
study in 2020 found the average cost for pharmacies to dispense each prescription was over $12.
24
Most of this cost is to support employment of pharmacists and technicians. Pharmacists report
inconsistent reimbursements make it challenging to adequately staff and resource retail pharmacies.
Pharmacists we interviewed noted there are some prescriptions they lose money on, some they make
a little profit on, and a small number they make a significant amount of profit on. PBMs can set
pharmacy reimbursement rates, which can vary significantly between drugs and pharmacy types.
Figure 13: CCOs reported spending $767 million on prescription drugs in 2021
Source: OHA publicly reported financial data
Other states have provisions in statute to address this issue. For instance, Kentucky prohibits PBMs
from reducing the amount reimbursed on a claim to effective rates; other states require the use of
National Average Drug Acquisition Costs as a basis for reimbursement when available. Arizona requires
PBMs to disclose the methodology for maximum allowable cost lists to provider pharmacies. Oregon
has no such provisions in statute. In some states these middlemen have been removed entirely from
their Medicaid coordinated care programs.
We analyzed 316,755 Medicaid claims to assess how pharmacy reimbursements may vary. Of the claims
we tested, 69% were far below the $12 average cited in the 2020 study. In our testing, more frequently
dispensed drugs — like metformin and omeprazole — tended to have much lower estimated pharmacy
profits than less frequently dispensed medications. For the 13 drugs tested, the average estimated
profit was $7.16 per claim, which likely is not enough to cover labor and other operating costs.
24
See the 2020 Cost of Dispensing Study commissioned by national pharmacy associations.
$226
$506
$523
$658
$698
$989
$251
$642
$649
$767
$949
$1,191
Emergency room
Mental health
Hospital- outpatient
Prescription drugs
Hospital- inpatient
Physician/professional services
$0 $1,200
●2019 ●2021
in millions

Oregon Secretary of State | Report 2023-25 | August 2023 | page 21
Overall, our analysis shows pharmacy reimbursements vary significantly between drugs, pharmacy
type, and PBM, as shown in Figures 14, 15, and 16. Pharmacies often lose money when filling certain
prescriptions and pharmacists have reported patients are sometimes turned away if a prescription will
cost the pharmacy too much money. In 2021, pharmacies dispensed over 12,000 acetaminophen
(Tylenol) prescriptions and, on average, made only $0.35 on each prescription. Pharmacies also
dispensed almost 71,000 albuterol sulfate prescriptions with a total estimated loss of over $1.3 million
or about $19 lost per prescription filled. Albuterol sulfate is an important lifesaving drug used to treat
breathing conditions due to asthma and allergies and is one of the most commonly dispensed
medications in Medicaid.
Figure 14: Pharmacy reimbursements vary widely between selected prescription drugs in 2021
Drug Number of
claims tested
Dollar amount
tested
Estimated total
pharmacy
profit/loss
Estimated average pharmacy
profit/loss per claim
Acetaminophen
(Tylenol)
12,178 $28,349 $4,269 $0.35
Albuterol Sulfate 70,955 $2,766,642 -$1,315,643 -$18.54
Amoxicillin 13,608 $51,462 $35,067 $2.58
Basaglar 45,767 $16,790,230 $219,619 $4.80
Biktarvy 6,195 $20,369,308 $192,792 $31.12
Budesonide and
Formoterol
Fumarate Dihydrate
14,924 $4,079,910 $865,952 $58.02
Buprenorphine and
Naloxone
26,101 $3,766,875 $1,238,829 $47.46
Eliquis 17,743 $8,457,352 $48,779 $2.75
Flovent 12,868 $3,357,669 $56,127 $4.36
Humira 3,947 $26,178,519 $25,836 $6.55
Metformin 26,184 $124,781 $62,680 $2.39
Omeprazole 47,812 $176,185 $117,167 $2.45
Trulicity 18,473 $15,267,881 $716,139 $38.77
Total 316,755 $101,415,163 $2,267,613
Source: Auditor analysis of OHA and PBM 2021 claims data and Oregon average acquisition cost data
Potential monopoly power may be putting patients and independent
pharmacies at risk
Pharmacies made profits on certain drugs, but those amounts vary widely and likely do not cover the
operating and overhead expenses incurred by the pharmacy. We found local, independent pharmacies
are more likely to be reimbursed less for prescriptions than national chain pharmacies. Figure 16
highlights the reimbursement disparity between pharmacy type and some name brand drugs per
prescription, like Humira and Biktarvy. For both drugs, the estimated profits for national chain
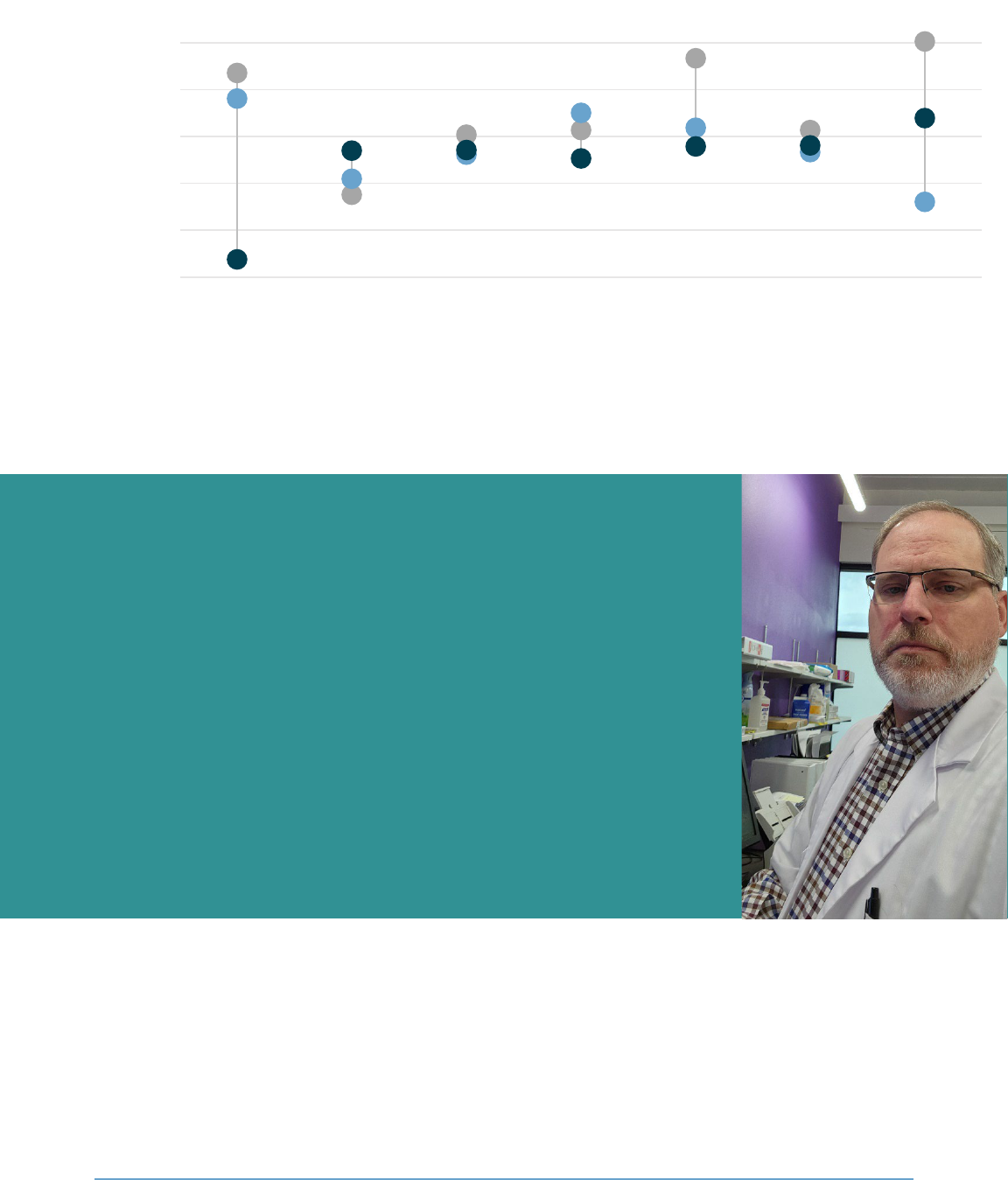
pharmacies are more than three times the amount that independent pharmacies made.
The Asthma and Allergy Foundation of America encourages states to adopt policies that promote access to
life saving medicine such as albuterol sulfate.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 22
In our testing, we estimate independent pharmacies, on average, are reimbursed less than national
chain pharmacies for most of the 13 drugs tested. For example, for each Eliquis prescription dispensed,
independent pharmacies lost on average about $3, whereas national chains made over $5. As we only
tested a small portion of pharmacy claims, we cannot conclude other drugs would show these same
disparities.
Figure 15: Medicaid PBMs consistently reimbursed pharmacies significantly below average acquisition cost
for more than 70,000 albuterol sulfate prescriptions in 2021
Source: Auditor analysis of OHA and PBM 2021 claims data and Oregon Average Acquisition Cost data
Figure 16: Estimated pharmacy profits for some brand name drugs differ significantly depending on
pharmacy type
Source: Auditor analysis of OHA and PBM 2021 claims data and Oregon Average Acquisition Cost data
-$32
-$24
-$17
-$14
-$13
-$9
($35)
($30)
($25)
($20)
($15)
($10)
($5)
$0
PBM 1 PBM 2 PBM 3 PBM 4 PBM 5 PBM 6
$58
$53
$27
$23
$7
$58
$48
$103
$45
$64
$61
$33
-$15
$48
$121
($25)
$0
$25
$50
$75
$100
$125
Budesonide Buprenorphine Humira Trulicity Biktarvy
Independent
National chain
Specialty/mail order
On average, the estimated profits for national chain and specialty/mail order pharmacies are more than
twice the amount independent pharmacies receive.
Oregon Secretary of State | Report 2023-25 | August 2023 | page 23
Figure 17: National and specialty/mail order pharmacies were reimbursed more than independent
pharmacies for most drugs tested
Source: Auditor analysis of OHA and PBM 2021 claims data and National and Oregon Average Acquisition Cost data
Long wait times, low pharmacy density, and unfair pharmacy reimbursements pose access issues for all
people in Oregon, not just Medicaid patients. Oregon’s Legislature should enact legislation prioritizing
fair and consistent reimbursement for community pharmacies.
“A little over a month ago our pharmacy got a desperate call from a woman from Madras. It was late
on Friday and her husband had been prescribed... a critical medicine to keep him out of the hospital.
She went to the only chain pharmacy open in town and they told her it would be Monday before
they could fill it. You see, both Bi-Mart and Hometown drugs had closed. Their pharmacy business
was booming but their reimbursements were too low to stay open. The remaining chain pharmacy in
town was so overwhelmed their wait times were measured in days. She called two chain pharmacies
in Redmond and could not get anyone to answer the phone. Again, reimbursements are too low to
keep adequate staffing, even to answer the phone. Then she called us. We told her to come right
away as it was close to closing and when she arrived, we quickly filled the prescription. She had lots
of questions and health care concerns, as she had not been able to speak to a pharmacist since
Hometown drugs had closed. When she left it was 30 minutes after closing and we felt happy that
we had helped someone in need that day. I then checked my reimbursement and found that I got
paid $26 below my acquisition price for that drug. This is not the value of the service we provided
that night. Independent pharmacies, in particular, have an important value to their communities, and
they should be paid fairly for that value.”
-
Kevin Russell, RPh, MBA, BCACP, Director of pharmacy at Prescryptive Health, oral
legislative testimony in support of HB3013 2023 regular session
Other states require PBMs to disclose certain information to better assess their value and
increase transparency
A lack of transparency in PBM processes has led many states to implement laws requiring PBMs to
disclose certain pricing and cost information. Information disclosed includes aggregated data on
($3.50)
($1.50)
$0.50
$2.50
$4.50
$6.50
Eliquis Acetaminophen Omeprazole Metformin Flovent Amoxicillin Basaglar
● Independent ● National chain ● Specialty/mail order

Oregon Secretary of State | Report 2023-25 | August 2023 | page 24
rebates, payments, and fees collected from drug manufacturers and pharmacies. Prior to 2023, PBM
reporting requirements have been established in 16 other states.
25
Figure 18: Before 2023, other states have passed laws requiring greater reporting for PBMs
Source: National Council of State Legislatures
To better understand this complex and often opaque system, policymakers should have information
available to them to make informed decisions and help determine whether PBMs and other key players
are providing benefits and delivering good value to Oregonians. Figure 19 compares Oregon to other
states that require PBMs to report certain financial information. Oregon’s initiatives were only recently
adopted in the 2023 session, but the Legislature should require PBMs to report admin fees and spread
pricing retained, as well as collecting de-identified data.
Figure 19: Oregon lacks some of the aggregated PBM transparency initiatives adopted in other states
OR CT GA IN IA LA MI MN MT NV NH NY TX UT VT VA WI
Drug rebates from drug
manufacturers or other
sources
● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ●
Drug rebates passed though
and/or retained
● ● ● ● ● ● ● ● ● ● ●
Admin fees from health plans ● ● ● ● ● ● ● ●
Admin fees from pharmacies ● ● ● ● ● ●
Admin fees from drug
manufacturers
● ● ● ● ● ● ● ● ● ● ●
All admin fees retained ● ● ● ● ● ●
Spread pricing retained ● ● ● ● ●
Data collection ● ● ● ●
Public reporting ● ● ● ● ● ●
25
In the 2023 session, the Oregon Legislature passed Senate Bill 192, which requires PBMs to submit some information annually
to DCBS.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 25
Source: States’ legislative websites
To illustrate the importance of capturing pricing information from PBMs, Figure 20 highlights how
reimbursement prices can vary dramatically. For Trulicity, a medication used for the treatment of type
2 diabetes, reimbursements resulted in estimated pharmacy losses of $58.82 per prescription to
estimated pharmacy profits of $53.29 per prescription.
Figure 20: Pharmacy reimbursement rates are inconsistent among PBMs in Medicaid
Drug NDC Month PBM Number
of units
Average Oregon
acquisition cost per unit
Estimated pharmacy
profit/loss
Prescription 1 Trulicity 2143380 Apr-21 PBM 1 2 $404.31 ($58.82)
Prescription 2 Trulicity 2143380 Apr-21 PBM 2 2 $404.31 ($16.58)
Prescription 3 Trulicity 2143380 Apr-21 PBM 3 2 $404.31 $9.57
Prescription 4 Trulicity 2143380 Apr-21 PBM 4 2 $404.31 $19.50
Prescription 5 Trulicity 2143380 Apr-21 PBM 5 2 $404.31 $53.29
Source: Auditor analysis of OHA and PBM 2021 claims data, NDC lists, and Oregon Average Acquisition Cost data
We analyzed CCO and PBM data for aggregate dispensing fees, administrative fees, drug rebates, and
other fees or payments received between 2017 and 2021. In aggregate, PBMs reported paying less in
dispensing fees to pharmacies than what CCOs reported paying to the same PBMs for dispensing fees,
for a difference of $612,000.
Drug rebate information was also analyzed but was not clear enough to make reasonable estimates and
rebate totals were less than our expectations. For example, aggregate drug rebates received in 2021
were reported to be about $7 million while CCOs reported spending over $700 million in pharmacy
benefits. Information was not confirmed with outside sources and there are likely other PBM revenue
sources we did not analyze. The difficulty with our analysis further provides evidence the system is
extremely complicated, and OHA does not have enough resources to reasonably determine whether
Medicaid PBMs are acting in the best interest of the program and patients under the current model.
States are switching PBM models to exert better control over Medicaid prescription drug
programs
Oregon’s Medicaid program currently uses a multiple PBM model. All 16 CCOs have the choice to
contract with OPDP to administer pharmacy benefits, or the CCOs can choose to contract with their
own PBM. In this model, CCOs pay their PBMs from capitation payments received from OHA. PBMs are
typically responsible for paying and processing pharmacy claims, developing or supporting the CCO’s
preferred drug list, negotiating with network pharmacies for lower price guarantees, and contracting
with drug manufacturers for drug rebates. This model has some tradeoffs.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 26
Pros: Current model Cons: Current model
•
Easier for CCOs to coordinate care
across pharmacy and medical benefits
•
Limited monitoring and knowledge of
PBM activities by OHA
• Drug rebates, which can lead to lower
costs, are not maximized
• Health equity concerns for members who
do not have consistent access
• Does not leverage purchasing power and
economies of scale
In an FFS model, prescription drug benefits would be administered by OHA. Pharmacy claims would be
processed by the contracted pharmacy benefit administrator, who currently performs those duties for
FFS claims. In this model, a uniform preferred drug list would be more efficient to implement,
prescribers would benefit from uniformity, and pharmacies would have more consistent
reimbursements
. West Virginia calculated $54.4 million in actual savings during the first year of their
transition to FFS.
26
While there is potential for cost savings, some states, like Florida,
27
have
determined a move to this model would be more costly.
Pros: FFS Cons: FFS
•
Potential decrease in capitation costs
• Potential increase in federal drug
rebates, which could lower costs
• Reimbursement consistency
• Increased transparency: State has
greater control of plan design and would
streamline monitoring efforts
• Statewide consistency in pharmacy
benefits administration
• Uniform formulary and coverage criteria
•
Leverages economies of scale
•
Requires development of IT solutions for
CCOs to access real-time pharmacy
claims and drug utilization for care
coordination
• Additional claims processing could strain
current IT infrastructure
• Potential loss of provider tax revenue
• Potential negative impacts to 340B
providers
28
A single PBM model has been used in other states to contract with one PBM for Medicaid coordinated
care or public employee health plans. For many health plans, Medicaid is not the only line of business,
and may include private insurance or Medicare. Health plans typically contract with one PBM for all lines
of business, and a move to a single PBM model may require plans to have contracts with multiple PBMs,
which could reduce some operational efficiency
. There is also a possibility some plans could withdraw
from Medicaid. In this model, Oregon could have some flexibility to maintain certain pharmacy
revenues, depending on the program structure, whereas an FFS model would not offer this kind of
flexibility.
26
See West Virginia’s 2019 Pharmacy Savings Report
27
See Florida’s 2020 PBM Pricing Practices in Statewide Medicaid Managed Care Program Report
28
Medicaid’s 340B program requires participating drug manufacturers to provide outpatient drugs to participating pharmacies
and entities at significantly reduced prices. State Medicaid programs are prohibited from billing manufacturers for rebates on
discounted medications under this program.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 27
Pros: Single PBM Cons: Single PBM
•
Potential decrease in capitation costs
• Increased transparency: Allows the state
to set contract parameters and would
streamline monitoring efforts
• Flexibility in pharmacy provider
reimbursements
• Uniform formulary and coverage criteria
• Leverages economies of scale
• Potential to preserve 340B pharmacy
revenues, depending on structure
•
Potential opposition from health plans
• Potential decreases in efficiency at the
health plan level, due to multiple lines of
business
• Potential loss of provider tax revenue
A PBM reverse auction is an online, competitive bidding process states can use to select a PBM to
manage prescription drug benefits. An auction starts with an opening bid and PBMs submit lower
counteroffers during multiple rounds of bidding. States achieve savings by forcing PBMs to offer the
same contract terms but at a lower price than their competitors. Reverse auctions have been known to
generate significant savings in various governmental procurements. New Jersey used a reverse auction
to select PBMs for public employee health plans and estimates the state will save $2.5 billion in drug
spending between 2017 and 2022.
29
While reverse auctions can lower costs for states, bid costs should
not be the only factor to consider. There is a risk PBMs might push cost savings to the pharmacy level,
which could result in low or unfair pharmacy reimbursements and ultimately exacerbate pharmacy
access issues. OHA staff reported several other risks relating to these types of procurements. For a
reverse auction to be successful it is essential that all requirements be clearly defined and
communicated. Any contract OHA considers should include pharmacy protections to ensure adequate
pharmacy access is available to all Medicaid patients.
Pros: Reverse auction Cons: Reverse auction
•
Potential cost savings
• Leverages free market competition
• Ability to define contract requirements
bidders must meet
•
Less flexible and additional costs may be
incurred with change orders
• Provider uncertainty between contract
terms
• Upfront procurement costs
In recent reports, the Prescription Drug Affordability Board and the Prescription Drug Price
Transparency Program mention centralized prescription drug purchasing programs. A centralized
prescription drug purchasing program would allow Oregon to privatize, standardize, and condense drug
programs currently run by multiple state insurers to improve cost-effectiveness while retaining
oversight, control, and accountability. This would also increase buying power by aggregating the
covered lives of those insurers and programs.
A few states currently have designated agencies that coordinate the purchase of drugs for state-run
facilities and programs. Programs in Washington and Louisiana purchase drugs related to certain
conditions like hepatitis C. Massachusetts’s program administers pharmacy services for corrections,
29
See the National Academy for State Health Policy’s article on PBM reverse auctions.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 28
developmental services, mental health, among others, but does not extend to Medicaid or state
employee insurers. One state that currently has a comprehensive purchasing program for all state
insurers and state-run facilities is California. In 2019, California’s governor consolidated the drug
purchasing of all state-run programs including the California Public Employees’ Retirement System,
Medicaid, and the California Corrections Department. The California Legislative Analyst’s Office has
estimated that the state could save “hundreds of millions of dollars” per year and the Governor’s office
estimates the savings at $150 million per year just for the state’s coordinated care entities. Using the
examples of other states, the legislature should explore establishing a state prescription drug
purchasing program to leverage lives covered and save tax-payer dollars.
OHA’s CCO contracting practices are not sufficient to ensure PBM
transparency and compliance
Moving to a single PBM or FFS structure will streamline OHA’s monitoring efforts and increase
transparency and efficiency; however, there are improvements OHA can implement in the meantime to
address high-risk areas such as rebate pass-through and spread pricing.
OHA relies on CCOs for monitoring and verification of PBM compliance. PBMs are subcontractors of
CCOs, and OHA does not have direct visibility into their performance, however CCO contracts allow
OHA to review records and information from the CCOs and any of their subcontractors. While OHA
improved CCO contracts in 2020 by adding PBM-specific requirements, more needs to be done to
ensure high-risk areas are monitored and contract provisions are enforced.
OHA made some improvements, but comprehensive contract provisions are still
needed
Prior to 2020, OHA’s contracts with CCOs had no PBM-specific requirements. Beginning in 2020, the
agency made significant improvements by adding certain terms to the CCO contracts. If a CCO chooses
to not use OPDP for pharmacy benefits, they must contractually require their chosen PBM to do the
following:
30
All contracts
• Pass through all rebates and other monies PBMs receive from drug manufacturers;
• Permit the CCO to perform an annual audit to ensure its PBM is compliant with
contractual requirements and is market competitive;
• Cooperate with the CCO to obtain a market check which clearly identifies data
used to compare to the PBM’s current performance;
• Renegotiate the contract if the market check determines the PBM’s performance is
1% behind the current market in cost savings;
• Make an attestation of financial and organizational accountability and a
commitment to the principle of transparency; and
• Provide full, clear, complete, and adequate disclosure to the CCO and OHA on the
services provided and all forms of income, compensation, and other payments
received or expects to receive under the subcontract with CCO.
30
This list is not all-inclusive. To see the full contract provisions, see OHA’s website.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 29
Pass-through contracts
• Pass through all costs so payments made to a pharmacy by the PBM agree to the
amounts the CCO paid to the PBM;
Pay-for-performance contracts
• PBM shares a portion of discounted drug costs and dispensing fees with CCOs,
based on over-performance of agreed upon performance measures.
Contracts permit CCOs to perform an annual audit of their PBMs. This optional provision does not
specify areas to be audited, which leaves it open to interpretation. Mandating CCOs to obtain a yearly
independent audit of high-risk areas could help give OHA reasonable assurance contract terms are
being met. It should be noted performing an annual audit does not replace a CCO’s responsibility for
ongoing monitoring of its PBMs.
OHA has the authority to employ a variety of sanctions on CCOs, but often opts for a more
collaborative approach. OHA reported examples of providing technical assistance, instead of corrective
action, to CCOs for instances of noncompliance. While cooperative, this method may not ensure timely
accountability. Without well-defined and communicated escalations steps, it may be difficult for OHA to
proactively enforce contract compliance. OHA should add specific contract language or reference
procedures that will go into effect if CCOs and PBMs are found to be out of compliance. A move to a
single PBM or FFS model would make monitoring easier for OHA.
The Barbara Roberts Human Services building, headquarters of the Oregon Health Authority | Source: Gary Halvorson, Oregon
State Archives

Oregon Secretary of State | Report 2023-25 | August 2023 | page 30
OHA performs minimal monitoring of CCOs and PBMs
OHA’s process for monitoring CCO contracts is coordinated by the agency’s quality assurance team.
Most contract deliverables are received by the quality assurance team and then sent to subject matter
experts within OHA for review. For PBM-specific deliverables, the subject matter experts are OPDP
staff.
31
This creates a potential conflict of interest as OPDP is a competing PBM.
Even with efforts to exercise professional independence, there still exists an appearance of competing
interests. Additionally, content monitoring of certain reports is not being done at all because of this
conflict of interest. OHA management indicated this situation is due to an ongoing lack of staff within
the quality assurance team that have experience with PBMs. They are considering adding personnel
with pharmacy experience to the team, which could move PBM contract provision review out of OPDP.
OHA should assign staff without a conflict of interest to monitor CCO and PBM compliance.
OHA has developed some controls specific to PBMs, which include contract reviews, market checks,
and pay-for-performance checks. These are improvements over past controls, but do not address
high-risk areas or provide reasonable assurance PBMs are compliant with contract provisions.
OHA reviewed contracts between CCOs and their PBMs after the new provisions were put in place. This
was done to ensure OHA’s PBM-specific requirements were incorporated. However, contract reviews
31
OPDP transitioned to ArrayRx on January 1, 2022. See OHA’s website for more information.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 31
are not contractually required on all amendments, nor have they been done on subsequent
amendments. OHA should require CCOs to submit all contracts and amendments with PBMs for review.
OHA also requires CCOs to complete a yearly market check, which is a comparison of PBM pricing with
the pricing of other comparable PBMs in the market. The intent of this check is to ensure CCOs
understand the need to hold PBMs accountable and to monitor for possible improvement. Data and
analysis from this report is presented to OHA at a summary level, but OHA does not review the
content, due to OPDP staff having a conflict of interest. Not monitoring this control leaves OHA with no
way of knowing if it is effective.
CCOs using a pay-for-performance contract must submit a quarterly report detailing administrative
and dispensing fees, number of claims, and amounts shared between the PBM and CCO, among other
data. OHA staff evaluate the information and send a summary letter to the CCOs and OHA
management for review. OHA intends for this review to identify whether generated PBM profits are
commensurate with the services provided; however, OHA has not required any CCO to act, even when
the evaluation shows the CCO is paying more than the market comparator.
Given the size and complexity of the Medicaid program, it is unreasonable to expect every contract
provision will be monitored in detail. However, for high-risk areas, OHA should focus resources to
provide effective monitoring tools and processes. This includes enforcing compliance with well-defined
escalation steps. Monitoring tools and their results should be evaluated on an ongoing basis and
feedback should be incorporated into the contracting process, which will help give reasonable
assurance CCOs and PBMs are complying.
Billions of dollars are spent on Medicaid. It is important the state take steps to ensure the program is
run efficiently and effectively to better serve people in Oregon. The current structure lacks
transparency and is too complex to efficiently measure value. The State should change the current
model and enact legislation that focuses on patient protections, pharmacy protections, and increasing
transparency in the prescription drug supply chain. Making these changes will help ensure the Medicaid
program is getting good value for pharmacy benefits, people have access to the same medications, and
Oregonians have access to community pharmacies.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 32
Recommendations
In order to have reasonable assurance PBMs are providing good value to Medicaid, we recommend
OHA:
1. Expand contract provisions to more proactively monitor and enforce contract compliance and
further develop monitoring processes that will give OHA reasonable assurance CCOs and PBMs
are in compliance. Consider the following:
• Require CCOs to obtain a yearly independent audit of their PBM for high-risk areas.
An independent audit could help give OHA reasonable assurance that CCOs and
their PBMs are in compliance. Note that a yearly audit should not replace a CCO’s
responsibility for on-going monitoring.
• Incorporate monitoring results into the contracting process to improve oversight
and program outcomes.
• Update the CCO contract to apply the review requirement to all CCO-PBM
amendments.
• Require PBM contracts to be pass-through.
2. Assign staff without a conflict of interest to monitor CCO and PBM compliance.
To increase transparency and streamline oversight for Medicaid pharmacy benefits, we recommend the
Legislature:
3. Implement a different PBM model in Medicaid coordinated care, such as a single PBM or Fee-For-
Service approach. If a single PBM model is chosen, explore using a reverse auction to choose the
vendor.
4. Mandate a universal preferred drug list and require uniform step and prior authorization criteria
for Medicaid coordinated care.
5. Implement uniform and fair pharmacy reimbursement policies for Medicaid coordinated care.
6. Include Medicaid PBMs in ORS 735.530.
7. Require PBMs operating in Oregon to act as fiduciaries to the health insurer/CCO they contract
with, and/or to the insured under a specific health plan.
8. Follow leading practices and require PBMs and CCOs to provide aggregate data to the
Department of Consumer and Business Services on a yearly basis which, at a minimum, details
the following:
• Total dispensing fees paid to both PBMs and pharmacies;
• Total admin fees obtained and retained from both manufacturers and health plans;
• Any monies obtained through spread pricing; and
• De-identified claims data that does not contain personally identifying information.
9. Study if the creation of a state prescription drug purchasing program would save tax-payer
dollars.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 33
Objective, Scope, and Methodology
Objective
1. Assess the value PBMs provide to the Medicaid program and identify opportunities for
improvement.
2. Determine whether OHA’s contract monitoring practices with CCOs and their PBMs are
adequate. Identify and report on potential conflicts of interest existing within the current CCO
and PBM structure.
Scope
Our audit tested coordinated care retail pharmacy claims from January 2021 to December 2021 and
aggregated rebate and payment information from PBMs and CCOs from January 2017 to December
2021. Our testing did not include prescription drugs given in a hospital setting or administered directly
by a physician.
Methodology
To address our objective, we conducted interviews with multiple stakeholders, including OHA, DCBS,
members of the Legislature, auditors from other state audit offices, staff from U.S. Senator Ron
Wyden’s office, community pharmacists, pharmacy industry associations, patient advocacy groups, and
PBM industry representatives. We also reviewed state rules and statutes related to the program and
our objectives.
We obtained final paid Medicaid claims data from OHA and pharmacy claims data from CCOs and their
corresponding PBMs which included fields like recipient ID, date of services, unique transaction number,
admin fees, dispensing fees paid to the pharmacy, ingredient cost paid to the pharmacy, and total
amount paid on the claim. The OHA data was joined to the PBM claims data. To assess the reliability of
the data, we traced a small sample of randomly selected claims to the originating system to provide
reasonable assurance the information we obtained was complete and accurate. Additionally, we
performed a variety of data verification techniques such as comparing control totals, verifying data
formatting, and logic tests. We did not verify the reported amounts paid by the PBMs to pharmacies
and it is possible that other transactions were not captured in our testing.
We judgmentally selected 13 different drugs to test based on the total dollars spent and number of
claims. The testing population was comprised of 18 different NDCs covering those drugs and we used
Oregon average acquisition data from Myers and Stauffer and National Average Drug Acquisition Cost
data to calculate the estimated pharmacy profit or loss. We tested 316,755 coordinated care retail
pharmacy claims covering $101,415,161 in costs.
As part of our audit, we calculated the estimated profit or loss pharmacies made on reimbursements
related to 13 different prescription drugs in 2021. The drugs selected for testing were combinations of
commonly prescribed and drugs with larger total aggregate payment amounts. To do this, we multiplied
the Oregon or national average acquisition cost for each drug by the quantity billed for the appropriate

Oregon Secretary of State | Report 2023-25 | August 2023 | page 34
time period and added any dispensing fees.
32
We then took this total and subtracted it from PBM
reported ingredient cost. A positive difference indicates the pharmacy likely made a profit, whereas a
negative amount indicates the pharmacy likely had a loss on the claim. We did not verify the
reimbursement amounts reported by PBMs to the pharmacies or the actual acquisition costs of the
drugs by pharmacies. It is likely some pharmacies with larger buying power had lower acquisition costs
than what was used in our calculations, and conversely some pharmacies likely had higher acquisition
costs.
We compared reimbursement amounts across PBMs and pharmacy types. Pharmacy types were
divided into four different categories: independent, national chain, specialty/mail order, and other. The
other category includes long-term care pharmacies and those housed in a health care setting. We also
received aggregate information from each CCO and PBM for drug rebates and other payments
received. We did not verify reported totals with outside entities.
In September 2022 and February 2023, we sent letters to the Legislature, as seen in Appendix C. While
this was not a real-time audit, we sent these letters to provide timely information to policymakers
ahead of and during legislative sessions.
Internal control review
We determined that the following internal controls were relevant to our audit objective.
33
• Risk Assessment
• We interviewed OHA management and staff to determine if risk areas were
assessed.
• Control activities
• We considered whether management has designed control activities to ensure CCO
and PBM compliance.
• Monitoring activities
• We considered whether management was effectively monitoring internal controls
to ensure CCO and PBM compliance.
Deficiencies with these internal controls were documented in the results section of this report.
We conducted this performance audit in accordance with generally accepted government auditing
standards. Those standards require that we plan and perform the audit to obtain sufficient, appropriate
evidence to provide a reasonable basis for our findings and conclusions based on our audit objectives.
We believe that the evidence obtained provides a reasonable basis for our findings and conclusions
based on our audit objectives.
We sincerely appreciate the courtesies and cooperation extended by officials and employees of OHA
during the course of this audit.
32
Myers and Stauffer, an OHA contractor, collects and reviews drug acquisition cost data provided by pharmacies enrolled in the
Medicaid FFS program. Cost information is collected via survey and rates are adjusted weekly. See the Myers and Stauffer
website
for posted rates.
33
Auditors relied on standards for internal controls from the U.S. Government Accountability Office, report GAO-14-704G.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 35
About the Secretary of State Audits Division
The Oregon Constitution provides that the Secretary of State shall be, by virtue of the office, Auditor
of Public Accounts. The Audits Division performs this duty. The division reports to the Secretary of
State and is independent of other agencies within the Executive, Legislative, and Judicial branches of
Oregon government. The division has constitutional authority to audit all state officers, agencies,
boards and commissions as well as administer municipal audit law.
Audit team
Ian Green, M.Econ, CGAP, CFE, CISA, CIA, Audit Manager
Kathy Davis, Senior Auditor
Bentley Walker, MSFA, CPCA, Staff Auditor
Wendy Kam, MBA, CFE, Staff Auditor

Oregon Secretary of State | Report 2023-25 | August 2023 | page 36
Appendix A: Glossary of Terms
Term Definition
340B pharmacy program
A federal program requiring drug manufacturers participating in Medicaid to
provide outpatient drugs to covered entities at significantly reduced prices.
State Medicaid agencies are prohibited from billing manufacturers for Medicaid
rebates for drugs dispensed to Medicaid patients that have already been
discounted under the 340B Program.
Actual acquisition cost
Actual acquisition cost is the state Medicaid agency's determination of
pharmacy providers' actual prices paid to acquire drug products marketed or
sold by a specific manufacturer and is the current Medicaid benchmark to set
payment for drug ingredients.
Administrative Fee
Administrative and service fees charged by pharmacy benefit managers to
manufacturers and to plan sponsors. These fees are typically a percentage of
the list (wholesale acquisition cost) price of a medicine.
Average wholesale price
The published list price for a drug sold by wholesalers to retail pharmacies and
nonretail providers. It is akin to a sticker price and used as a starting point for
negotiation for payments to retail pharmacies.
Brand Drug
Branded products are not generic drugs or products. A brand can be an
innovator (first-in-class) or not. It is protected by a patent or has an expired
patent.
Clawback
A contractual provision that reclaims money already paid out. As applied to
independent pharmacies, clawback clauses appear in many pharmacy benefit
manager contracts in the form of direct and indirect remuneration fees, as well
as generic effective rate and brand effective rate post-adjudication
recoupments.
Coordinated Care Organization (CCO)
A network of all types of health care providers (physical, behavioral, and dental
care providers) who work together in their local communities to serve people
who receive coverage under Medicaid. CCOs focus on prevention and helping
people manage chronic conditions.
Department of Consumer and Business
Services (DCBS)
Oregon’s largest business regulatory and consumer protection agency. The
department administers state laws and rules to protect consumers and
workers in the areas of workers’ compensation, occupational safety and health,
financial services, insurance and building codes. The department serves as an
integrated umbrella agency over most state functions affecting businesses in
order to improve efficiency and effectiveness.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 37
Dispensing fee
The professional fee which:
(1) Is incurred at the point of sale or service and pays for costs in excess of the
ingredient cost of a covered outpatient drug each time a covered outpatient
drug is dispensed;
(2) Includes only pharmacy costs associated with ensuring that possession of
the appropriate covered outpatient drug is transferred to a Medicaid
beneficiary. Pharmacy costs include, but are not limited to, reasonable costs
associated with a pharmacist's time in checking the computer for information
about an individual's coverage, performing drug utilization review and
preferred drug list review activities, measurement or mixing of the covered
outpatient drug, filling the container, beneficiary counseling, physically
providing the completed prescription to the Medicaid beneficiary, delivery,
special packaging, and overhead associated with maintaining the facility and
equipment necessary to operate the pharmacy.
Drug manufacturer
Any person or firm which manufactures, compounds, or packages a drug for
wholesale in the pharmaceutical form in which it is sold by retail to the public.
Drug rebates
These are provided by manufacturers and are typically based on the ability of a
payer to move market share for the manufacturer’s product. Rebates are
confidential. Rebates are billed periodically by the insurer or PBM based on
drug utilization subject to the rebate. Rebates allow the manufacturer to retain
a high list price (which can be important to the manufacturer so any US price
that might wind up in the reference pricing system of another country is high).
Fee-for-service (FFS)
A method/model in which doctors and other health care providers are paid for
each service performed directly by OHA (not through a CCO).
Fiduciary
A fiduciary is a person or entity who holds a legal or ethical responsibility to act
in the best interests of their clients.
Generic drug
Competitors to a branded product that has an expired patent. Generics are
considered identical to the brand product.
Mail order pharmacy
A pharmacy located within a U.S. jurisdiction whose primary business is to
dispense a prescription drug or device pursuant to a valid prescription drug
order and to deliver the drug or device to a patient via the United States
Postal Service, a common carrier, or a delivery service. “Mail order pharmacy”
includes a pharmacy that does business via the internet or other electronic
media.
Maximum allowable cost
The average price of all the multisource drugs in a group. The frequency the
maximum allowable cost is recalculated is at the discretion of the payer. The
multi-source drugs to which a maximum allowable cost is applied is also at the
discretion of the payer.
National Average Drug Acquisition
Cost
A national average of the prices at which pharmacies purchase a prescription
drug from manufacturers or wholesalers, including some rebates.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 38
National Drug Code (NDC)
The unique three segment number assigned to each drug subject to
commercial distribution and which is used and serves as a universal product
identifier.
Oregon Prescription Drug Program
Established in 2007, the purpose of the program is to purchase prescription
drugs or reimburse pharmacies for prescription drugs to receive discounted
prices and rebates, make prescription drugs available at the lowest possible
cost to participants in the program and to maintain a list of prescription drugs
recommended as the most effective prescription drugs available at the best
possible prices.
Pass-through contract
The PBM passes through the amount charged by the pharmacy to the health
insurer. Typically, PBMs will charge a per claim administrative fee in this type of
contract.
Patient steering
Some PBMs require contracted health plan beneficiaries to visit affiliated
pharmacies, or pharmacies in which they have an ownership interest including
retail, mail-order, or specialty.
Pharmacy auditing Audits of pharmacies by PBMs to detect fraud, waste, and abuse.
Pharmacy benefit manager (PBM)
PBM clients are health plans. PBMs handle some or all the pharmacy benefit for
health plans (formulary design, cost sharing and tiers, pharmacist networks
and contracts, price concession negotiation with manufacturers). PBMs may
own mail order pharmacies and/or specialty pharmacies. Unless the PBM owns
a pharmacy, it is not part of the drug distribution/supply chain.
Pharmacy collective groups
This term refers to either Group Purchasing Organization or Pharmacy Services
Administration Organizations.
Pharmacy network
A pharmacy network is a list of pharmacies or pharmacists that a health plan or
PBM has contracts with to provide prescription drug services to its members.
Preferred drug list
A list of prescription drugs that are identified by a contractor’s Pharmacy and
Therapeutics Committee as the preferred drugs for prescriptions within a
therapeutic drug class.
Reverse auction
An online, competitive bidding process states can use to select a PBM to
manage prescription drug benefits. An auction starts with an opening bid and
PBMs submit lower counteroffers during multiple rounds of bidding. States can
achieve savings by requiring PBMs to offer the same contract terms but at a
lower price than their competitors.
Specialty drug
A drug that is costly, requires special supply chain features (such as freezing or
cold storage), typically indicated for a small group of patients, and where the
patients may need special case management services. This is the broadest
definition. There is no single agreed-upon definition, so sometimes specialty
drug will only mean high-cost. For instance, specialty drugs in the Medicare
Part D program are only defined by cost.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 39
Specialty pharmacy
Specialty pharmacies are different from traditional retail pharmacies in
coordinating many aspects of patient care and disease management. They can
deliver medications with special handling, storage, and distribution
requirements with standardized processes that permit economies of scale.
Many specialty and mail order pharmacies are vertically integrated with PBMs
and insurers.
Spread pricing
The difference between what a health plan pays the PBM and the amount that
the PBM reimbursed the pharmacy for a beneficiary’s prescription.
Unaffiliated pharmacies
A network pharmacy that directly or indirectly does not control, is not
controlled by, and is not under common control with, a PBM.
Vertical integration
A business organization strategy in which all stages of production of a good or
service are controlled by one company. Example: A corporation owns a PBM
that works with outside insurers to send patients to retail, mail-order, or
specialty pharmacies that the corporation also owns.
Wholesale acquisition cost
The price the wholesaler pays the manufacturer and is generally considered
the “list” price. However, this price is not what wholesalers pay for drugs.
Wholesalers
In a simple distribution system, the wholesaler is the first purchaser of a drug
product — direct from the manufacturer. Wholesalers buy very large quantities
and then resell either direct to provider-purchasers (like a large health system,
pharmacy or pharmacy chain), or resell to smaller, regional distributors for
regional or local distribution to retail pharmacies and hospitals.

Oregon Secretary of State | Report 2023-25 | August 2023 | page 40
Appendix B: Detailed Oregon pharmaceutical supply chain diagram
34
34
*HealthShare has multiple subcontractors, each with their own pharmacy benefit relationship. These include OHSU, Providence, Kaiser, CareOregon, OptumRx, and Legacy/Pacific Source.

Shemia Fagan Secretary of State
Cheryl Myers Deputy Secretary of State, Tribal Liaison
Kip Memmott
MA, CGAP, CRMA
Audits Director
255 Capitol St. NE Suite 180
Salem OR 97310
(503) 986-2255
sos.oregon.gov/audits
September 14, 2022
Representative Nancy Nathanson
Oregon Legislature
900 Court St. NE
Salem, OR 97301
Dear Representative Nathanson:
In response to your request, we are providing information about leading practices adopted in other
states regarding pharmacy benefit managers (PBMs). This letter identifies potential opportunities for
legislative action related to Oregon PBMs.
States have implemented these practices in their Medicaid program or public employee health plans
and are taking a varied approach in adopting PBM models. Each approach has different costs and
benefits and there is no perfect solution.
Health equity concerns
Pharmacy closures have negatively impacted Oregon’s rural and frontier communities. In addition,
studies show pharmacy closures affect poor communities and disproportionately impact Black and
Latino communities.
1
Pharmacies are a critical element of health care and are important in promoting
health outcomes for the people of Oregon.
2,3
Pharmacists are ideally positioned in their community to
address gaps in care by collaborating with other health care providers, which can help eliminate health
disparities.
4
For these reasons, steps should be considered to protect Oregon pharmacies.
Pharmacy protections
Pharmacy audits, regulations, and appeals
PBMs regularly audit pharmacies to identify improper payments and verify the patient received the
correct medication and dosage. There is a risk PBMs could abuse this process to recoup money from
expensive prescriptions for minor administrative errors that do not harm the patient
. Some states have
1
Guadamuz JS, Wilder JR, Mouslim MC, Zenk SN, Alexander GC, Qato DM. Fewer pharmacies in black and hispanic/latino
neighborhoods compared with white or diverse neighborhoods, 2007–15.
2
Qato DM, Alexander GC, Chakraborty A, Guadamuz JS, Jackson JW. Association Between Pharmacy Closures and Adherence to
Cardiovascular Medications Among Older US Adults.
3
Hillier-Brown, F., Bambra, C., Thomson, K. et al. The effects of community pharmacy public health interventions on population
health and health inequalities: a systematic review of reviews protocol.
4
Westberg SM, Sorensen TD. Pharmacy-related health disparities experienced by non-English-speaking patients: impact of
pharmaceutical care.
Appendix C: Interim Letters to the Legislature

Oregon Secretary of State | September 2022 | page 2
implemented laws to ensure pharmacies are audited fairly and given a process for appeal. Oregon has
some laws in place for pharmacy audit protections, though it is important to note current PBM
regulatory statutes do not apply to Medicaid managed care PBM relationships or where insurers act as
their own PBMs.
5
Despite these laws, pharmacists have stated ongoing predatory practices are occurring and noted the
existing appeals process is cumbersome and time-consuming
. The Oregon Department of Consumer
and Business Services can investigate complaints submitted by pharmacies against PBMs for some
issues, including violation of MAC (maximum allowable cost) pricing requirements, predatory pharmacy
audit practices, and violation of “gag clause” rules. Examples: Delaware
and Ohio.
Fair pharmacy reimbursement
The number of pharmacies has been falling across the nation, and local, independent pharmacies have
been impacted the most. Having access to a local pharmacy is critical in addressing health inequities
and supporting positive health outcomes at the individual and population level.
6
Low reimbursement
rates are often cited as a key factor for pharmacy closures and some states have provisions for fair
reimbursement. Approaches from other states include prohibiting PBMs from reducing the amount
reimbursed on a claim to effective rates or requiring the use of National Average Drug Acquisition Costs
as a basis for reimbursements when available
. Some states require PBMs to disclose the methodology
for maximum allowable cost lists to provider pharmacies, and others have limited fees a PBM can levy
on a pharmacy
. Examples: Arkansas, Kentucky, and West Virginia.
Enrollee protections
Uniform formulary
A formulary, or preferred drug list, is a list of prescription drugs that are covered under a health plan.
Health plans use formularies as a way to negotiate drug rebates and encourage providers to prescribe
those medications
. Uniform formularies support administrative simplicity for providers and consistent
drug coverage for patients.
Some states with uniform formulary legislation for Medicaid require the same formulary for all managed
care health plans
, while others have chosen to adopt uniform formularies for only certain classes of
drugs
. See the FFS (Fee-For-Service) model below for more information on states that have entirely
carved out pharmacy benefits from managed care, which would also produce a uniform formulary and
coverage criteria for all Medicaid members
. A shift to this structure should include considerations for
phase-in implementation that would give prescribers and enrollees time to manage prescription
changes, a mechanism for CCOs (coordinated care organizations) to access pharmacy claims, and drug
utilization for care coordination
. Examples: Mississippi, Ohio, and Washington.
5
Oregon Revised Statutes 735.530 – 735.552
6
Oregon’s Prescription Drug Program (OPDP) has a statutory charge to coordinate comprehensive prescription benefit services
in Oregon and supports fair reimbursement practices through its ‘Critical Access Pharmacy’ designation. See Oregon Revised
Statutes 414.312 and Oregon Administrative Rules 431-121

Oregon Secretary of State | September 2022 | page 3
Figure 1: Many states have adopted policies for uniform formularies for all or some drug classes in Medicaid
managed care
Source: Kaiser Family Foundation
Increased transparency
Reporting and transparency requirements
Most states have implemented laws requiring PBMs to disclose certain pricing and cost information
such as data on rebates, or payments and fees collected from drug manufacturers and pharmacies. This
helps health regulators and health plans to make informed decisions regarding whether PBMs are
providing benefits and delivering good value. Examples: Louisiana and Minnesota
.
Figure 2: 32 states have passed laws requiring greater transparency or reporting for PBMs
Source: National Council of State Legislatures

Oregon Secretary of State | September 2022 | page 4
Medicaid prescription benefit models
Multiple PBMs
Currently, Oregon’s Medicaid program uses a multiple PBM model. All 16 CCOs have the choice to
contract with the Oregon Prescription Drug Program to administer pharmacy benefits, or the CCOs can
choose to contract with their own PBM.
7
In this model, CCOs pay their PBMs from capitation payments
received from the OHA (Oregon Health Authority)
. PBMs are typically responsible for paying and
processing pharmacy claims, developing or supporting the CCO’s formulary, negotiating with network
pharmacies for lower price guarantees, and contracting with drug manufacturers for drug rebates
.
Pros Cons
•
Easier for CCOs to coordinate care across
pharmacy and medical benefits
• Outsources processing and payment of
pharmacy claims
•
Limited monitoring and knowledge of PBM
activities by OHA
• Drug rebates, which can lead to lower costs,
are not maximized
• Health equity concerns for members who do
not have consistent access
• Does not leverage purchasing power and
economies of scale
Reverse auction
A PBM reverse auction is an online, competitive bidding process states can use to select a PBM to
manage prescription drug benefits. An auction starts off with an opening price and PBMs submit lower
counteroffers during multiple rounds. States achieve savings by forcing PBMs to offer the same
contract terms but at a lower price than in preliminary rounds of bidding. Reverse auctions have been
known to generate significant savings in various governmental procurements. New Jersey uses reverse
auctions to select PBMs for public employee health plans and estimates the state will save $2.5 billion
in drug spending between 2017 and 2022. While reverse auctions can lower costs for states, bid costs
should not be the only factor to consider. There is a risk PBMs might push cost savings to the
pharmacy level, which could result in low or unfair pharmacy reimbursements and ultimately exacerbate
pharmacy access issues. Examples: New Jersey and Maryland
.
Pros Cons
•
Potential cost savings
• Leverages free market competition
• Ability to define contract requirements
bidders must meet
•
Less flexible and additional costs may be
incurred with change orders
• Provider uncertainty between contract
terms
Fee-for-Service (FFS)
In an FFS model, prescription drug benefits would be administered by OHA. Pharmacy claims would be
processed by the contracted pharmacy benefit administrator, who currently performs those duties for
FFS claims. In this model, a uniform formulary would be more efficient to implement, prescribers would
benefit from uniformity, and pharmacies would have more consistent reimbursements
. West Virginia
7
As of 2022, only one CCO has chosen to use the Oregon Prescription Drug Program to administer pharmacy benefits.

Oregon Secretary of State | September 2022 | page 5
calculated $54.4 million in actual savings during the first year of the transition to FFS.
8
While there is
potential for cost savings, some states, like Florida,
9
have determined a move to this model would be
more costly. There may also be negative fiscal impacts to 340B pharmacies. Examples: Missouri,
West
Virginia, and Wisconsin.
Pros Cons
•
Potential decrease in capitation costs
• Potential increase in federal drug rebates,
which could lower costs
• Reimbursement consistency
• Increased transparency-state has greater
control of plan design and would streamline
monitoring efforts
• Statewide consistency in pharmacy benefits
administration
• Uniform formulary and coverage criteria
•
Leverages economies of scale
•
Requires development of IT solutions for
CCOs to access real-time pharmacy claims
and drug utilization for care coordination
• Additional claims processing could strain
current IT infrastructure
• Potential loss of provider tax revenue
• Potential negative impacts to 340B providers
Single PBM
A single PBM model has been used in other states to contract with one PBM for Medicaid managed care
or public employee health plans. For many health plans, Medicaid is not the only line of business, and
may include private insurance or Medicare. Health plans typically contract with one PBM for all lines of
business, and a move to a single PBM model may require plans to have contracts with multiple PBMs,
which could reduce some operational efficiency
. There is also a possibility some plans could withdraw
from Medicaid. In this model, Oregon could have some flexibility to maintain 340B pharmacy revenues,
depending on the program structure, whereas an FFS model would not offer this kind of flexibility.
Examples: Ohio and Kentucky
.
Pros Cons
•
Potential decrease in capitation costs
• Increased transparency- allows the state to
set contract parameters and would
streamline monitoring efforts
• Flexibility in pharmacy provider
reimbursements
• Uniform formulary and coverage criteria
• Leverages economies of scale
• Potential to preserve 340B pharmacy
revenues, depending on structure
•
Potential opposition from health plans
• Potential decreases in efficiency at the
health plan level, due to multiple lines of
business
• Potential loss of provider tax revenue
8
See West Virginia’s 2019 Pharmacy Savings Report
9
See Florida’s 2020 PBM Pricing Practices in Statewide Medicaid Managed Care Program Report

Oregon Secretary of State | September 2022 | page 6
When considering new legislation or adoption of new models, it is important to consider both
quantitative and qualitative impacts to enrollees, the program, and key stakeholders like local
pharmacies. We suggest you reach out to OHA while drafting legislation to discuss the impacts to the
programs they administer. We hope you find value in this communication
.
We appreciate OHA’s time and collaboration during our ongoing audit of PBMs
. We plan on issuing our
audit report next year, which will provide additional details around these leading practices as well as
risk areas and important background information. If you have any questions, please contact Audit
Manager Ian Green at (971) 239-7934.
Sincerely,
Kip Memmott
Director, Audits Division
Oregon Secretary of State
CC: Patrick Allen, Director, Oregon Health Authority
CC: Representative Rob Nosse; Senator Elizabeth Steiner Hayward
CC: Chairs of the Interim Senate and House Committees on Health Care; Joint Committee on Legislative
Audits
CC: Gina Zejdlik, Governor’s Office

Shemia Fagan Secretary of State
Cheryl Myers Deputy Secretary of State, Tribal Liaison
Kip Memmott
MA, CGAP, CRMA
Audits Director
255 Capitol St. NE Suite 180
Salem OR 97310
(503) 986-2255
sos.oregon.gov/audits
February 6, 2023
House Committee on Behavioral Health and Health Care
Oregon Legislature
900 Court St. NE
Salem, OR 97301
Dear Chair Nosse and Vice-Chairs Goodwin and Nelson:
We are providing information about leading practices related to Pharmacy Benefit Manager (PBM)
transparency reporting requirements. We are providing this information now because the Oregon
Legislature is considering legislation on this topic, and our audit of Medicaid PBMs will be released after
legislative deadlines elapse. Such practice is in accordance with Government Auditing Standards:
“To be of maximum use, providing relevant evidence in time to respond to officials of the audited
entity, legislative officials, and other users’ legitimate needs is the auditors’ goal ... During the audit, the
auditors may provide interim reports of significant matters to appropriate entity and oversight officials.
Such communication alerts officials to matters needing immediate attention and allows them to take
corrective action before the final report is completed.”
1
Increased transparency
Reporting requirements
Policymakers across the country are taking a multifaceted approach in tackling rising prescription drug
costs. One area that has received national attention in the past few years is the part PBMs play in
health care. PBMs play a key role in the complex pharmacy process and can provide a wide array of
services, such as processing claims, performing drug utilization review, creating formularies, and
negotiating contracts between health plans, manufacturers, and pharmacies. Over time, their roles and
responsibilities have changed from mostly claims adjudication to having significant influence over many
aspects of the prescription drug system.
A lack of transparency in PBM processes has led many states to implement laws requiring PBMs to
disclose certain pricing and cost information, such as data on rebates or payments and fees collected
from drug manufacturers and pharmacies. PBM reporting requirements have been established in 16
states, as shown in Figure 1.
1
U.S. Government Accountability Office, Yellow Book Generally Accepted Government Auditing Standards 9.17

Oregon Secretary of State | February 2023 | page 2
Figure 1: Other states, but not Oregon, have passed laws requiring PBMs to report information
2
Source: National Council of State Legislatures
Many of Oregon’s efforts to lower prescription drug costs have been targeted at drug manufacturers
and not PBMs. To better understand this complex and often opaque system, policymakers should have
information available to them to make informed decisions and help determine whether PBMs and other
key players are providing benefits and delivering good value to Oregonians. Figure 2 details some of the
requirements for which 16 other states require their PBMs to submit information.
Figure 2: Rebates, administrative fees, and spread pricing are common elements reported in other states
OR
CT
GA
IN
IA
LA
MI
MN
MT
NV
NH
NY
TX
UT
VT
VA
WI
Drug rebates from drug
manufacturers or other
sources
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
Drug rebates passed though
and/or retained
●
●
●
●
●
●
●
●
●
●
Admin fees from health plans
●
●
●
●
●
●
●
●
Admin fees from pharmacies
●
●
●
●
●
●
Admin fees from drug
manufacturers
●
●
●
●
●
●
●
●
●
●
All admin fees retained
●
●
●
●
●
Spread pricing retained
●
●
●
●
●
Data collection
●
●
●
●
Public reporting (in aggregate)
●
●
●
●
●
●
Source: National Council of State Legislatures
2
Note this figure differs from the map in the letter sent to Representative Nathanson in September 2022. The previous map
included other transparency measures adopted by states that are not reflected in the map above, which only includes PBM
reporting requirements. See the National Conference of State Legislatures website for more information.
Oregon Secretary of State | February 2023 | page 3
To illustrate why it is important to capture pricing information from PBMs, we have included a table
highlighting how pricing can vary dramatically between PBMs and the pharmacies that dispense the
drugs. For Trulicity, a medication used for the treatment of type 2 diabetes, our audit work has
identified Medicaid reimbursement rates resulted in estimated pharmacy losses of $58.82 per
prescription dispensed to estimated pharmacy profits of $53.29 per prescription dispensed.
Figure 3: Pharmacy reimbursement rates are inconsistent between PBMs in Medicaid
Drug
NDC
Month
PBM
Number of
units
Average Oregon
acquisition cost
Estimated pharmacy
profit/loss
Prescription 1
Trulicity
2143380
Apr-21
PBM1
2
$404.31
($58.82)
Prescription 2
Trulicity
2143380
Apr-21
PBM2
2
$404.31
($16.58)
Prescription 3
Trulicity
2143380
Apr-21
PBM3
2
$404.31
$9.57
Prescription 4
Trulicity
2143380
Apr-21
PBM4
2
$404.31
$19.50
Prescription 5
Trulicity
2143380
Apr-21
PBM5
2
$404.31
$53.29
Source: Preliminary auditor analysis of OHA and PBM Medicaid data
Our audit work is focused on PBMs within the Medicaid program and not those regulated by the
Department of Consumer and Business Services (DCBS). It is important to note current statutes define
PBMs as an organization that contracts with pharmacies on behalf of an insurer offering a health
benefit plan, a third-party administrator, or the Oregon Prescription Drug Program established in ORS
414.312.
3
DCBS does not consider Medicaid PBMs to fall under that definition and are therefore not
subject to most statutory requirements. As a result, Medicaid PBMs are not regulated by DCBS, and
statutory changes are required if the legislative intent is to capture pricing information from all PBMs
operating in the state. Medicaid serves one in three people in Oregon and the current limited statutory
definition provides an incomplete picture of all PBMs operating in the state.
When considering new legislation, it is important to consider both quantitative and qualitative impacts
to enrollees, the program, and key stakeholders. We suggest reaching out to DCBS and OHA and other
stakeholders while drafting legislation to discuss the impacts to the programs they administer. We
hope you find value in this communication and would be happy to answer any questions you have.
We plan on issuing our audit report later this year, which will provide additional details around leading
practices as well as risk areas and important background information. If you have any questions, please
contact Audit Manager Ian Green at (971) 239-7934.
Sincerely,
Kip Memmott
Director, Audits Division
Oregon Secretary of State
CC: James Schroeder, Director, Oregon Health Authority
CC: Representative Nancy Nathanson; Representative Rob Nosse; Senator Elizabeth Steiner
CC: Chairs of the Senate Committee on Health Care; Joint Committee on Legislative Audits
CC: Andrea Cooper, Governor’s Office
3
See ORS 735.530 for the full definition of a pharmacy benefit manager.

August 16, 2023
Kip Memmott, Director
Secretary of State, Audits Division
255 Capitol St. NE, Suite 180
Salem, OR 97310
Dear Mr. Memmott:
This letter provides a written response to the Audits Division’s final draft audit report titled
Patients and Independent Pharmacies are Harmed by Poor Accountability and
Transparency from Medicaid Pharmacy Benefit Managers (PBMs).
The Oregon Health Authority (OHA) appreciates the role of the Secretary of State Audits
Division in providing oversight of Oregon’s State-funded programs on behalf of taxpayers
and the people we serve. The scope of this audit was limited to retail or community
pharmacy claims from January 2021 to December 2021 and aggregated rebate and
payment information from PBMs and CCOs from January 2017 through December 2021.
The audit did not look at prescription drugs given in a hospital setting or administered by a
practitioner. The objective was stated to be:
1. Assess the value PBMs provide the Medicaid program and identify opportunities for
improvement.
2. Determine whether OHA’s contract monitoring practices with CCOs and their PBMs
are adequate. Identify and report on potential conflicts of interest existing within the
current CCO and PBM structure.
The SOS office made two recommendations to OHA and outlined several
recommendations to the legislature. First, OHA would like to offer comments on the
recommendations made to the legislature. The audit’s focus is on PBMs’ role in the CCOs’
delivery of pharmacy benefits for Medicaid recipients statewide. However, there are
recommendations that have broad applicability when considering PBM regulation
generally. This is particularly true for recommendations #6 (“Including PBMs that serve
Medical Assistance programs directly or indirectly in OAR 735.530”) and #8 (“Require
annual reporting of PBMs and CCOs to DCBS”). OHA agrees that PBMs serving CCOs
should abide by the same set of rules governing them in the commercial entities that they
serve. OHA also agrees that reporting requirements to DCBS should be aligned across all
lines of business conducted by PBMs. This can be a tool to improve the visibility of
regulators and improve state agency responsiveness in terms of monitoring and regulating
PBMs practices. Recommendation #4 proposes there be a uniform preferred drug list
(PDL) and prior authorization (PA) criteria for Medicaid. It seems that the SOS Audit team
feels that change is due in this area to address inequities and ensure a truly portable
OFFICE OF THE DIRECTOR
Tina Kotek, Governor
500 Summer St. NE E-20
Salem, OR 97301
Voice: 503-947-2340
Fax: 503-947-2341
www.oregon.gov/oha

August 16, 2023
Page 2 of 4
pharmacy benefit for Medicaid recipients. OHA has heard favorable patient and
practitioner feedback around creating a single PDL and uniform PA criteria for all Medicaid
recipients. OHA would like to better understand what is envisioned with recommendation
#5, “Implement a fair and uniform reimbursement policy for CCOs,” as this could mean
various things to different readers. One such uniform reimbursement policy could be a
single PBM, which could bring uniform reimbursement to pharmacies. However, policies
such as requiring minimum dispensing fees and designating critical access pharmacies
could be interpreted as addressing fair reimbursement as well as sustaining access to
pharmacy services.
Additionally, with respect to recommendation #9, “Study if the creation of a state
prescription drug purchasing program would save tax-payer dollars,” OHA suggests
exploring the Oregon Prescription Drug Program (OPDP)/ArrayRx as a possible PBM
model and/or solution for Medicaid coordinated care programs. The OPDP/ArrayRx
Steering Committee members have collectively over 150 years of pharmacy experience
and the program has a 20-year history of cost savings. Via an interstate cooperative
agreement, Oregon, Washington, Nevada and soon, Connecticut utilize OPDP/ArrayRx to
effectively leverage all participating programs’ purchasing power. Rolling Medicaid
managed care PBM services into OPDP/ArrayRx would effectively double this program’s
current purchasing power and, in turn, reduce the administrative costs for Oregon and the
other interstate cooperative members.
Finally, recommendation #3 recommends exploring reverse auctions. These are costly, as
a vendor must first be selected to conduct the auction, and it is likely that PBMs would
reduce payment to pharmacies to make their reverse auction bid work. This, in turn, would
worsen the pharmacy access issues Oregon is currently experiencing. Further, reverse
auctions do not abide by Oregon procurement statutes and rules and would violate specific
procurement requirements. Conversely, OPDP/ArrayRx has demonstrated savings and
effectiveness within the state over the past 20 years, whereas reverse auctions do not
have this long-standing history.
Below is our detailed response to each OHA recommendation in the audit.
RECOMMENDATION 1
Expand contract provisions to more proactively monitor and enforce contract
compliance and further develop monitoring processes that will give OHA reasonable
assurance CCOs and PBMs are in compliance. Consider the following:
• Require CCOs to obtain a yearly independent audit of their PBM for high-risk
areas. An independent audit could help give OHA reasonable assurance that
CCOs and their PBMs are in compliance. Note that a yearly audit should not
replace a CCO’s responsibility for on-going monitoring.
• Incorporate monitoring results into the contracting process to improve oversight
and program outcomes.
• Update the CCO contract to apply the review requirement to all CCO-PBM
amendments.
• Require PBM contracts to be pass-through.

August 16, 2023
Page 3 of 4
Agree or Disagree
with
Recommendation
Target date to
complete
implementation
activities
Name and phone number of
specific point of contact for
implementation
Agree
01/01/2025
David Inbody
Narrative for Recommendation 1
OHA conducts an annual CCO contract restatement process. These recommended
considerations can be included in the 2025 restatement, which will begin in February 2024.
The specific considerations identified are partially represented in the existing CCO
contract.
• Yearly independent audit – Currently a subcontractor performance audit is required
for all high-risk subcontractors, which would include PBMs. The audits are
conducted by CCOs. In addition, an annual PBM market check is conducted by a
third party on behalf of the CCOs, for those not using OPDP. The contract could be
changed to include an independent audit for PBMs; this would incur additional costs
for all CCOs.
• Incorporate monitoring results into contracting process – The addition of Quality
Assurance staff with the background and experience to enable contract oversight
without the appearance of conflict of interest.
• Update CCO contract to apply review requirements to all CCO-PBM amendments
or contract renewals. Currently, this requirement only applies to pay-for-
performance PBM subcontracts. This could be changed as part of the next contract
restatement process to apply to all CCO-PBM amendments and contract renewals.
OHA’s CCO PBM readiness review includes a market check requirement, which could be
expanded to include an audit of the PBM annually. The third-party nature of this market
check requirement along with an annual audit would indeed give more assurances to OHA
and better position CCOs to oversee their PBMs’ behavior. As noted in the report, CCOs
are permitted to utilize OPDP/ArrayRx as their PBM; only one CCO currently elected to
utilize this option. It is important to note that OPDP/ArrayRx do perform a third-party review
and market check for all programs participating in interstate cooperative agreement.
Additionally, OPDP/ArrayRx conducts quarterly sample audits to ensure claim submission
and payment accuracy for a diverse subset of claims processed for our participating
programs. Annually, ArrayRx completes an audit representing in excess of 50% of its total
claims. This audit assesses the accuracy of 100% of claims that are processed by our
PBM for these programs (as opposed to audits that are conducted in quarterly PBM
audits). The most recent audit results showed that OPDP/ArrayRx PBM had a 99.99%
accuracy rate.
We agree that all subsequent contract amendments between PBMs and CCOs should be
subject to OHA review and approval. This can be in place for the 2025 contract. OHA also
agrees that PBM contracts should be pure pass-through contracts moving forward and will
change the PBM readiness review process to reflect this. To successfully address the
additional analytics and oversight necessary, it would be helpful to find a path to effectively
resource OHA. A recent legal settlement between Oregon and a PBM highlights the need
to have a dedicated resource that can assist with audits, oversight, and review of PBM

August 16, 2023
Page 4 of 4
activities performed to best serve our Medicaid members. It would benefit all parts of OHA
and other agencies to have a centralized pharmacy purchasing resource that can manage
multiple pharmacy claims databases and ensure all Oregon agencies stay ahead of market
changes that happen quickly and covertly within the pharmacy space.
RECOMMENDATION 2
Assign staff without a conflict of interest to monitor CCO and PBM compliance.
Agree or Disagree
with
Recommendation
Target date to
complete
implementation
activities
Name and phone number of
specific point of contact for
implementation
Agree
07/01/2024
Trevor Douglass
Narrative for Recommendation 2
OHA agrees with this recommendation; however, staffing challenges have led to
leveraging staff who have knowledge and expertise in the PBM contracting and pharmacy
space. It was noted by OHA staff and confirmed by DOJ that a potential conflict of interest
existed if OPDP/ArrayRx staff—who currently have this expertise—were to review CCOs’
PBM materials. After the first CCO PBM readiness review, OHA did take steps to mitigate
the conflict of interest by removing OHA’s Director of Pharmacy Policy, Programs and
Purchasing from all subsequent reviews. However, the remaining staff member—the
OPDP/ArrayRx Operations Manager—still has a conflict of interest. It should be noted that
other divisions within OHA and other Oregon agencies struggle to identify independent
pharmacy analytic, policy, and purchasing resources, which suggests these resources are
needed statewide. OHA intends to pursue adding resources to ensure availability of staff
without a conflict of interest.
For any questions, please contact:
• Trevor Douglass, DC, MPH – Pharmacy Policy, Programs and Purchasing Director -
• David Inbody – Medicaid Coordinated Care Organization Operations Manager –
• April Gillette, MPH, CVT, CPHQ – Governance & Process Improvement Director –
Sincerely,
Dave Baden
Interim Director
EC: Kristine Kautz, OHA Deputy Director
Janell Evans, Interim OHA Chief Financial Officer
Dana Hittle, OHA Medicaid Director
Shawna McDermott, Interim OHA Health Systems Division Director
Chris DeMars, OHA Office of Delivery System Innovation Director

