
Behav Ecol Sociobiol (2004) 55:569–573
DOI 10.1007/s00265-003-0745-6
ORIGINAL ARTICLE
Debra A. Lewkiewicz · Marlene Zuk
Latency to resume calling after disturbance in the field cricket,
Teleogryllus oceanicus
, corresponds to population-level differences
in parasitism risk
Received: 17 July 2003 / Revised: 1 October 2003 / Accepted: 15 December 2003 / Published online: 22 January 2004
Springer-Verlag 2004
Abstract A possible parasitoid-evasion behavioral adap-
tation is examined in male field crickets, Teleogryllus
oceanicus, from three Hawaiian islands where parasitoid
prevalence varies naturally among islands. Ormia ochra-
cea, the parasitoid fly that parasitizes T. oceanicus on
these islands, uses male calling song to locate its hosts.
We used laboratory-reared males from three Hawaiian
islands to determine if there are population differences in
the time it takes for calling males to resume calling after a
standardized disturbance. Males follow the expected
pattern; males from the island with the greatest risk of
parasitism have the longest latency to resume calling, and
males from the island with the least risk of parasitism
have the shortest latency to resume calling. Results are
discussed in the context of behavioral adaptations to
differing parasitism levels, and trade-offs between natural
and sexual selection.
Keywords Parasitoid · Teleogryllus oceanicus · Ormia
ochracea · Risk aversion · Calling song
Introduction
Many aspects of reproduction have been shown to
increase individuals’ risk of predation and/or parasitism
(Endler 1987; Magnhagen 1991; Forsgren and Magn-
hagen 1993; Zuk and Kolluru 1998). For acoustically
signaling animals, such as crickets, in which males call to
attract mates, this trade-off between survival and repro-
duction can be particularly important, since such calls, by
their very nature, must be conspicuous (Cade 1975; Bell
1979; Gray and Cade 1999; Kolluru 1999; Lehmann et al.
2001). In such cases, we expect to find selection for
behaviors that allow for increased reproductive effort
during times of low predation risk and decreased repro-
ductive effort during times of high predation risk. These
behavioral adaptations must entail early detection and
evasion of predators (Miller and Surlykke 2001). Such
behaviors may involve increasing the use of cover when
risk is high (Banks 2001), or using indications of predator
proximity as cues to cease displaying (Spangler 1984;
Jennions and Backwell 1992).
The field cricket Teleogryllus oceanicus was intro-
duced to three of the Hawaiian islands (Oahu, the Big
Island of Hawaii, and Kauai) at least 150 years ago
(Kevan 1990; Zuk et al. 1993). Native to Australia and the
Pacific, T. oceanicus is subject to parasitism in Hawaii by
the introduced parasitoid fly, Ormia ochracea (Zuk et al.
1993, 1998). O. ochracea females home in on the calling
song of T. oceanicus and deposit larvae on and around the
cricket (Cade 1975). These larvae burrow into the body of
the cricket and feed on thoracic and abdominal muscle
tissue for 7–10 days, after which they emerge from the
body cavity of the cricket, killing it within hours (Adamo
et al. 1995). Previous work has documented several
responses to parasitism in T. oceanicus, including changes
in song structure, the diel distribution of calling, and the
age structure of parasitized populations (Zuk et al. 1993;
Simmons and Zuk 1994; Rotenberry et al. 1996). The
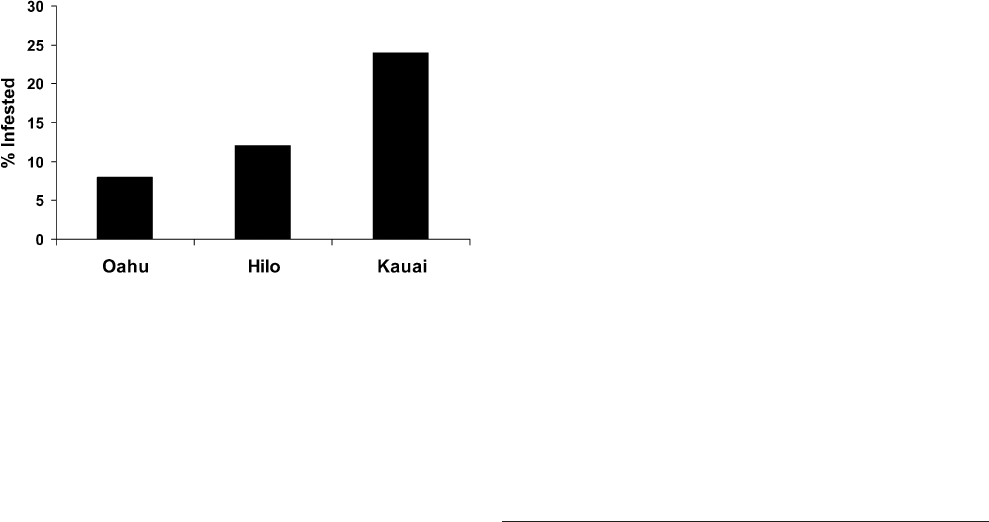
three islands differ in the prevalence of fly infestation,
with about 8%, 12%, and 25% of calling males harboring
larvae on Oahu, the Big Island of Hawaii, and Kauai,
respectively (Zuk et al. 1993; Fig. 1). The prevalences
have remained stable over repeated sampling in different
seasons and years (Zuk, unpublished data). These differ-
ences in the likelihood of becoming parasitized are
reflected in the degree of change in the song from
unparasitized populations (Rotenberry et al. 1996).
Here we examine another possibly adaptive response
to degree of risk: the latency to resume calling after a
disturbance. Hedrick (2000) demonstrated that call laten-
cy was an effective measure of caution for another field
cricket with varying degrees of intrinsic risk. Puffs of air
Communicated by D. Gwynne
D. A. Lewkiewicz (
)
) · M. Zuk
Department of Biology,
University of California,
Riverside, CA 92521, USA
e-mail: [email protected]
Tel.: +1-909-8277021
Fax: +1-909-7874286
were used as disturbances because: (1) these have been
shown to elicit escape responses in crickets (Bentley
1975; Gnatzy and Kmper 1990; Gras and Hrner 1992;
Kanou 1999), and (2) crickets’ cercal wind receptors are
extremely sensitive (Kumagai et al. 1998), making it
possible that crickets could use such a disturbance in the
early detection of approaching parasitoid flies. Crickets
are capable of detecting and acting evasively toward the
airborne disturbances created by the wing beats of a
flying parasitoid wasp (up to 3 cm away) (Gnatzy and
Heußlein 1986; Gnatzy and Kmper 1990). O. ochracea
have a fairly straight flight path and land, on average,
8.2 cm away from their target, although many will land
directly on their target (Mller and Robert 2001). This
means that crickets may have the opportunity to detect
flying natural enemies.
Cessation of calling has been described as an anti-
predator startle response in other orthoptera (Sales and
Pye 1974; Spangler 1984; Faure and Hoy 2000). It is
likely that using silence as an avoidance mechanism is a
useful tool for evading parasitism in Hawaiian T.
oceanicus, since O. ochracea are extremely adept at
localizing cricket song (Mason et al. 2001). Previous
studies indicate that O. ochracea are capable of locating
sources of cricket calling song even if such calls are
fragmented (Mller and Robert 2002) or if calls cease
after flies have initiated search flight (Mller and Robert
2001). Importantly, even though flies show such extraor-
dinary homing capabilities, their preference and accuracy
at locating call sources diminishes with increasing call
fragmentation and periods of silence (Mller and Robert
2001, 2002). Thus, inserting such periods of silence into
calling songs after the detection of a possible danger cue
is potentially a critically important behavior for male
crickets exposed to fly parasitism. However, periods of
silence likely reduce males’ chances of attracting mates,
as female field crickets use calling song to locate males,
and they tend to prefer calls of long duration (Wagner
1996) and few interruptions (Hedrick 1986). Thus, while
male populations on all islands should face sexual
selection pressures to minimize periods of silence, they
should also face opposing natural selection pressures
imposed by O. ochracea to increase periods of silence
after a disturbance. The strength of these natural selection
forces should vary in accordance to the risk of parasitism
experienced by male crickets on each island. We therefore
predicted that the latency to resume calling after a
disturbance would correspond to the level of parasitism
rates among the three islands. Even if T. oceanicus males
cannot specifically detect approaching O. ochracea,we
would still expect males from populations exposed to high
risk to display a higher degree of general caution than
males from lower-risk populations when exposed to risk
signals. Since air disturbances cause escape responses in
crickets (Bentley 1975; Gnatzy and Kmper 1990; Gras
and Hrner 1992; Kanou 1999), it is likely that males
from high-risk populations will respond more cautiously
(with longer latencies to resume calling) than males from
lower-risk populations.
Methods
All males were taken from laboratory stocks that were collected
from Oahu, Kauai, and Hilo on the Big Island of Hawaii between
1993 and 1999. New individuals were added to all stocks every 1–
2 years to minimize the effects of inbreeding. Details of collection
sites are given in Zuk et al. (1993, 2001). Prior to isolation, crickets
were housed in mixed-sex and mixed-age containers in 30C
incubators on a 12:12 light:dark cycle. Crickets were given ad
libitum water and Flukers Cricket Feed and provided with
cardboard egg crates for shelter. Males were isolated in separate
containers (8.5 cm in diameter) at least 12 h before use. Isolated
males were given cat food and water ad libitum, along with a piece
of cardboard egg crate for shelter during the light cycle when
experiments were not in progress. Trials took place between 2 and
12 h after the onset of the dark cycle. Cardboard egg crates were
removed from all isolated male containers before the onset of trials.
Trials were performed blind, with the identity of the population
being tested unknown until after the test was complete. Single
individual male containers were placed within Styrofoam boxes to
prevent visual disturbances. A turkey baster (a kitchen tool with a
rubber bulb on one end of a plastic tube) was attached to the outside
of the Styrofoam box and was used to generate a puff of air as a
standardized disturbance. The puff of air was transferred into the
individual male’s container via a 30-cm piece of plastic tubing that
connected the plastic tube of the baster to the inside of the male’s
container.
Males called continuously (with no breaks longer than 2 s) for at
least 2 min before a puff of air was delivered into the container by
gently depressing the bulb on the baster. In all but one case, the
male immediately stopped calling after the puff of air was
delivered. The latency for males to resume calling continuously,
after this cessation, was timed with a stopwatch. A male was
considered to have resumed calling continuously if he called for at
least 1 min with no longer than 2-s breaks in the call. If a male did
not resume calling within 600 s, he was assigned a conservative
latency score of 600 s.
Males were measured three times. Hilo and Kauai males were
tested in an environmental growth chamber at 28.4C. This
chamber was unavailable for the Oahu males, which were tested
in a room lacking temperature regulation with temperatures varying
from 20.0C to 28.3C (mean=24.7C). Although all males were
not tested simultaneously, this should not present a problem since
Fig. 1 Prevalence of Ormia ochracea larvae in calling male
Teleogryllus oceanicus from Kauai, Oahu, and Hilo on the island
of Hawaii. Details of cricket and parasitoid collection in Zuk et al.
(1993, 1995). Number of males examined: 79 for Kauai, 118 for
Hilo, and 126 for Oahu. Kauai samples were collected in 1993 and
1995, Hilo samples in 1992 and 1993, and Oahu samples in 1993
and 1994
570

T. oceanicus are not seasonal, and our adult lab colonies are
continuously replenished with newly molted adults. Thus, colonies
consistently have a mix of young and old adults. Low temperatures
could decrease calling activity. If this were the case, we would
expect low temperatures to increase latencies to resume calling in
Oahu males. However, a Kendall rank-order correlation showed no
significant association between temperature and latency to call in
Oahu males (t=0.46, P=0.32). Furthermore, any association
between latency to call and temperature would only make our
comparison of latency scores across populations more conservative,
since we predicted that Oahu males should have the shortest
latencies to call, and cooler temperatures should tend to increase
their latencies. Other studies indicate that low temperatures tend to
alter various call components (such as chirp duration, chirp rate,
pulse length, pulse rate, and wing stroke rate) in such a way that
reduces general call activity (Prestwich and Walker 1981; Pires and
Hoy 1992; Ciceran et al. 1994; Martin et al. 2000).
A Jonckheere test for ordered alternatives (Siegel and Castellan
1988) utilized only one measurement per individual; the shortest
latency was used for males in all populations. Shortest latencies
were used to minimize any slight affects of temperature that may
have been experienced by Oahu individuals. Note that the
Jonckheere test for ordered alternatives can be used in situations
similar to those in which one would use a Kruskal-Wallis rank
ANOVA. The difference is that, while a Kruskal-Wallis tests the
hypothesis that one of k independent samples is different from at
least one other, the Jonckheere test for ordered alternatives is used
when one has an a priori hypothesis about the order of sample
medians (Siegel and Castellan 1988), such as a test of dosage
dependence effect of a drug. Similarly, we had an a priori
hypothesis about the order of the population medians. The P-value
associated with the Jonckheere test reflects the probability not only
that there are significant differences among populations, but also
that these differences occur in the predicted order. A chi-square
goodness of fit analyses was performed that utilized all three
observations per individual.
Results
Males did not differ in their degree of call fragmentation
[measured as the number of short (<2 s) periods of silence
in the call counted within 2 min] before disturbance
(Kruskal-Wallis c
2
2
=0.194, P=0.918). Therefore the dif-
ferences among populations in latencies to call were
unlikely a result of pre-existing differences in call
fragmentation in the absence of disturbance. Latencies
to resume calling were not normally distributed and
differed significantly among populations (J*=4.86,
P<0.0001; Fig. 2). Consistent with our prediction, median
latencies were longest for Kauai males (n=30, median=
20.33 s), intermediate for Hilo males (n=39, median=
10.35 s), and shortest for Oahu males (n=34, median=
2.74 s).
A chi-square goodness of fit test on the number of
times, out of three, an individual resumed calling within
the 600 s allotted also corroborated this pattern (c
2
6
=17.161, P<0.0087; Fig. 3). Although some of the cells in
the contingency table had expected values of less than 5,
we followed Rosenthal and Rosnow’s (1991) suggestion
that such a criterion is unnecessarily strict when n is large
(n=88, in this case). In any case, when we collapsed cells
to correct for this issue such that the number of
individuals that resumed calling all three times and the
number of individuals that never resumed calling were
compared among populations, the same population pat-
tern remained (c
2
2
=10.757, P<0.0046).
Discussion
The latencies to resume calling after a disturbance among
the three Hawaiian populations followed the expected
pattern. Males from Oahu, where the risk of parasitism is
lowest, had the significantly shortest latencies to call
(Fig. 2), and were most likely to resume calling within the
allotted period of time after being disturbed (Fig. 3).
Males from Hilo, where the risk of parasitism is
intermediate, had intermediate latencies to call (Fig. 2).
Unlike Oahu males, which always resumed calling at least
Fig. 2 Box plot of latencies to resume calling following a
disturbance in male T. oceanicus from Oahu (n=34), Hilo (n=39),
and Kauai (n=30). Boxes enclose 50% of the observations, and lines
within boxes represent median values. Bars indicate 85% quantiles.
Although males were measured more than once, only the shortest
latency time (s) for each male was used to create this box plot
Fig. 3 Comparison of the percentage of T. oceanicus males from
Oahu (n=30), Hilo (n=30), and Kauai (n=28) that resumed calling
0, 1, 2, or 3 times in the allotted time (600 s) out of three
disturbance trials (c
2
6
=20.351, P<0.0024)
571

twice out of three trials, 10% of Hilo males either never
resumed calling or only resumed once out of three times
(Fig. 2). Males from Kauai, where parasitism risk is the
greatest, had the longest latencies to call. Over 30% of
Kauai males either never resumed calling or only called
once out of three trials (Fig. 3). Because the crickets were
all from laboratory populations subject to the same
standardized disturbance, the difference among popula-
tions appears to be a trait under genetic control, rather
than being a function of the environment in which the
animals find themselves.
Other acoustically signaling animals have been shown
to use silencing as an effective defense against predators
(Spangler 1984; Jennions and Backwell 1992). The lesser
wax moth, Achroia grisella, and the bush katydid, Insara
covilleae, both use acoustic calls to attract mates.
Spangler (1984) demonstrated that both of these insects
momentarily ceased calling when they heard either real or
pre-recorded predatory bat vocalizations. However, such
silencing behavior may not always be effective in
avoiding predation or parasitism. O. ochracea in Florida
that home in on male Gryllus rubens calling songs often
remain near the source of the call for more than 5 min
after the call has ceased, and sometimes remain for over
20 min (Walker 1993). This may represent a behavioral
counter-adaptation by the flies. If female O. ochracea
employ such prolonged waiting behavior in the three
Hawaiian islands discussed in this paper, then males with
short or even intermediate latencies to call may have little
chance at evading these parasitoid flies. However, it is
unlikely that a female fly would continue to wait near a
silenced cricket if another nearby male cricket was
calling. Thus, the effectiveness of using silence as an
avoidance mechanism may be dependent upon the
proximity of other calling males. Other researchers have
also expressed doubt about the effectiveness of silencing
behavior for orthopteran hosts of O. ochracea. Mller and
Robert (2002) demonstrated that even though call frag-
mentation in G. rubens reduces female O. ochracea
accuracy at localizing the sound source, this reduction in
accuracy is quite small (a difference of 5–6 cm). How-
ever, the periods of silence the authors inserted into a
calling song to create a “highly” fragmented call were
quite small and were intended to represent natural
variation in an undisturbed call, not a call that has been
fragmented due to a disturbance. Thus, the amount of
fragmentation observed in our study could have a much
larger effect in fly landing accuracy.
Although cessation of calling for certain periods of
time may not always prove an effective means of
avoiding parasitism (Bullock 1984), it is one of several
lines of defense that a male cricket can use to decrease its
chances of infection. Periods of silence may make it more
difficult for flies to accurately locate their hosts (Mller
and Robert 2001), and because of this, a nearby
unsilenced male may become a more desirable target.
During periods of silence, male crickets are also better
able to listen and focus on any other external cues in their
environment that could predict an approaching natural
enemy (Faure and Hoy 2000). Males in this study were
confined to relatively small containers, thus limiting their
movement, and were visually isolated from the observer;
however the observer was able to audibly detect instances
when males jumped after disturbance, and this activity
was not uncommon (personal observation). In the field,
cessation of calling in response to air disturbances may
frequently be coupled with a jumping or running
response, which has been demonstrated in another Gryllid
(Gras and Hrner 1992). Together, silencing and loco-
motion may prove a very effective escape response,
especially since O. ochracea respond to call cessation by
maintaining their original flight path (Mller and Robert
2001).
Male T. oceanicus face a trade-off between survival
and reproduction. Here, we have demonstrated that males
from populations with differing degrees of parasitism
differ in the degree of caution they exhibit after a
disturbance, in terms of their latencies to resume calling.
Males from highly parasitized islands take longer to
resume calling after a disturbance than males from less
parasitized islands. While males with long periods of
silence following a disturbance may reduce their chances
of parasitism, they are also likely to suffer a decrease in
reproductive success if they are less likely to attract
females (Hedrick 1986; Wagner 1996).
Acknowledgements We are grateful to the students who help
maintain the cricket colonies. M.Z. was supported by grants from
the National Science Foundation and from the Academic Senate of
the University of California, Riverside, Calif. J. Calkins, S.N.
Gershman, S.P. Scott, and A.M. Stoehr made useful comments on a
previous version of the manuscript. The experiments described in
this paper comply with the current laws of the United States of
America.
References
Adamo SA, Robert D, Hoy RR (1995) Effects of a tachinid
parasitoid, Ormia ochracea, on the behaviour and reproduction
of its male and female field cricket hosts (Gryllus spp.). J Insect
Physiol 41:269–277
Banks PB (2001) Predation-sensitive grouping and habitat use by
eastern grey kangaroos: a field experiment. Anim Behav
61:1013–1021
Bell PD (1979) Acoustic attraction of herons by crickets. J N Y
Entomol Soc 87:126–127
Bentley D (1975) Single gene cricket mutations: effects on
behaviour, sensilla, sensory neurons, and identified interneu-
rons. Science 187:760–764
Bullock TH (1984) Comparative neuroethology of startle, rapid
escape, and giant fiber-mediated responses. In: Eaton RC (ed)
Neural mechanisms of startle behavior. Plenum, New York, pp
1–13
Cade W (1975) Acoustically orienting parasitoids: fly phonotaxis to
cricket song. Science 190:1312–1313
Ciceran M, Murray A-M, Rowell G (1994) Natural variation in the
temporal patterning of calling song structure in the field cricket
Gryllus pennsylvanicus: effects of temperature, age, mass, time
of day, and nearest neighbour. Can J Zool 72:38–42
Endler JA (1987) Predation, light intensity and courtship behaviour
in Poecilia reticulata (Pisces: Poeciliidae). Anim Behav
35:1376–1385
572
Faure PA, Hoy RR (2000) The sounds of silence: cessation of
singing and song pausing are ultrasound-induced acoustic
startle behaviors in the katydid Neoconocephalus ensiger
(Orthoptera; Tettigoniidae). J Comp Physiol A 186:129–142
Forsgren E, Magnhagen C (1993) Conflicting demands in sand
gobies: predators influence reproductive behaviour. Behaviour
126:125–135
Gnatzy W, Kmper G (1990) Digger wasp against crickets. II. An
airborne signal produced by a running predator. J Comp Physiol
A 167:551–556
Gnatzy W, Heußlein R (1986) Digger wasp against crickets. I.
Receptors involved in the antipredator strategies of the prey.
Naturwissenschaften 73:212–215
Gras H, Hrner M (1992) Wind-evoked escape running of the
cricket Gryllus bimaculatus. J Exp Biol 171:189–214
Gray DA, Cade WH (1999) Sex, death, and genetic variation:
natural and sexual selection on cricket song. Proc R Soc Lond B
Biol Sci 266:707–709
Hedrick AV (1986) Female preferences for male calling bout
duration in a field cricket. Behav Ecol Sociobiol 19:73–77
Hedrick AV (2000) Crickets with extravagant mating songs
compensate for predation risk with extra caution. Proc R Soc
Lond B Biol Sci 267:671–675
Jennions MD, Backwell PRY (1992) Chorus size influences on the
anti-predator response of a Neotropical frog. Anim Behav
44:990–992
Kanou M, Ohshima M, Inoue J (1999) The air-puff evoked escape
behaviour of the cricket Gryllus bimaculatus and its compen-
sational recovery after cercal ablations. Zool Sci 16:71–79
Kevan DKM (1990) Introduced grasshoppers and crickets in
Micronesia. Bol San Veg 20:105–123
Kolluru GR (1999) The effects of an acoustically-orienting
parasitoid fly (Ormia ochracea) on reproduction in the field
cricket, Teleogryllus oceanicus: a trade-off between natural and
sexual selection. PhD dissertation, University of California,
Riverside, Calif.
Kumagai T, Shimozawa T, Baba Y (1998) Mobilities of the cercal
wind-receptor hairs of the cricket, Gryllus bimaculatus. J Comp
Physiol A 183:7–21
Lehmann GUC, Heller K-G, Lehmann AW (2001) Male bushcrick-
ets favoured by parasitoid flies when acoustically more
attractive for conspecific females (Orthoptera: Phanopteridae/
Diptera: Tachinidae). Entomol Gen 25:135–140
Magnhagen C (1991) Predation risk as a cost of reproduction.
Trends Ecol Evol 6:183–186
Martin SD, Gray DA, Cade WH (2000) Fine-scale temperature
effects on cricket calling song. Can J Zool 78:706–712
Mason AC, Oshinsky ML, Hoy RR (2001) Hyperacute directional
hearing in a microscale auditory system. Nature 410:686–690
Miller LA, Surlykke A (2001) How some insects detect and avoid
being eaten by bats: tactics and countertactics of prey and
predator. Bioscience 51:570–581
Mller P, Robert R (2001) A shot in the dark: the silent quest of a
free-flying phonotactic fly. J Exp Biol 204:1039–1052
Mller P, Robert R (2002) Death comes suddenly to the
unprepared: singing crickets, call fragmentation, and parasitoid
flies. Behav Ecol 13:598-606
Pires A, Hoy RR (1992) Temperature coupling in cricket acoustic
communication. J Comp Physiol A 171:69–78
Prestwich KN, Walker TJ (1981) Energetics of singing in crickets:
effect of temperature in three trilling species (Orthoptera:
Gryllidae). J Comp Physiol A 143:199–212
Rosenthal R, Rosnow RL (1991) Essentials of behavioural
research: methods and data analysis, 2nd edn. McGraw-Hill,
New York
Rotenberry JT, Zuk M, Simmons LW, Hayes C (1996) Phonotactic
parasitoids and cricket song structure: an evaluation of
alternative hypotheses. Evol Ecol 10:233–243
Sales G, Pye D (1974) Ultrasonic communication by animals.
Chapman and Hall, London
Siegel S, Castellan NJ Jr (1988) Nonparametric statistics for the
behavioural sciences, 2nd edn. McGraw-Hill, New York
Simmons LW, Zuk M (1994) Age structure of parasitized and
unparasitized populations of the field cricket Teleogryllus
oceanicus. Ethology 98:333–340
Spangler HG (1984) Silence as a defence against predatory bats in
two species of calling insects. Southwest Nat 29:481–488
Wagner WE (1996) Convergent song preferences between female
field crickets and acoustically orienting parasitoid flies. Behav
Ecol 7:279–285
Walker TJ (1993) Phonotaxis in female Ormia ochracea (Diptera:
Tachinidae), a parasitoid of field crickets. J Insect Behav
6:389–410
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by
predators and parasitoids. Q Rev Biol 73:415–438
Zuk M, Simmons LW, Cupp L (1993) Calling characteristics of
parasitized and unparasitized populations of the field cricket
Teleogryllus oceanicus. Behav Ecol Sociobiol 33:339–343
Zuk M, Simmons LW, Rotenberry JT (1995) Acoustically-orienting
parasitoids in calling and silent males of the field cricket
Teleogryllus oceanicus. Ecol Entomol 20:380–383
Zuk M, Rotenberry JT, Simmons LW (1998) Calling songs of field
crickets (Teoleogryllus oceanicus) with and without phonotac-
tic parasitoid infection. Evolution 52:166–171
Zuk M, Rotenberry JT, Simmons LW (2001) Geographical
variation in calling song of the field cricket Teleogryllus
oceanicus: the importance of spatial scale. J Evol Biol 14:731–
741
573
