
Need help or have questions?
Contact Patient Services toll-free at 1.877.593.6421
or visit myheartmonitor.com for online support.
Scan the QR code to view how to setup your ePatch.
Patient education guide
Extended Holter
ePatch

2
Table of contents
Before you begin
About our service 3
What to expect during service 4
Getting started 5
Good to know
Record symptoms 9
Changing the patch 10
Important information 13
Showering instructions 14
Returning the equipment 15
Travel information 16
Appendix
ePatch addendum to the patient education guide 18
Terms and conditions of the BioTelemetry
service agreement 21
Notice of condentiality and privacy practices 22
Patients’ rights and responsibilities 26
For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
3
About our service
Your physician has prescribed the Philips extended Holter – ePatch for you. ePatch
continuously records and stores heartbeats that are analyzed by cardiac technicians
at Philips. Clinical reports are made available to your healthcare provider at the end
of service.
To get started, review the important information in this guide or visit
myheartmonitor.com.
If you have any questions about your monitoring service or billing, please contact us:
Patient Services: 1-877-593-6421 (toll-free)
Email: customerservice@gobio.com
Hours: Mon–Fri: 8am–8:30pm ET; Sat–Sun: 8am–4:30pm ET

4
What to expect during service
Contacting you
Before, during or after your service,
we may contact you for any of the
following reasons:
• Confirm insurance information
• Assist in starting service
• Troubleshooting
• On behalf of your physician
Please note, we will not contact
you regarding heart-related
ndings, unless specically
instructed by your physician.
Billing for service
Charges are incurred when you
begin monitoring. During or after
your service, your insurance company
will send you an Explanation of
Benets (EOB). An EOB from your
insurance carrier is not a bill.
You will be responsible for any
out-of-pocket cost associated
with deductibles, co-insurances,
etc. If there is a balance due,
you will receive a statement
from Philips for your portion.
If you have any questions
regarding the balance due,
please refer to your statement for
contact information.
Prompt return of all equipment
The device and kit components
enclosed are the property of Philips
and must be returned immediately
upon completion of service.
Failure to return may result in
delayed delivery of the nal test
results and a bill for the cost of
the device.
Device return instructions are
located in this guide. Please refer
to the table of contents for the
page number.
Before you begin

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
5
Getting started
• It is important to
prepare your skin
before applying
the device. If hair
is on your chest in
the indicated area
(red circle), shave hair
using a razor.
Step 1: Prepare your skin
A
Left side
• Wash the area (red
circle) with soap and
water.
• Dry thoroughly with
a towel.
• Do not apply lotions
or oils.
B
Left side
• Gently rub the
skin using the tool
provided (see image
below).
Note: This step may
help inproviding a
better ECG signal.
C
Left side
Getting started
6
• Remove a patch from
the patch pouch.
• Place the sensor
*
into
the patch and press
down FIRMLY to snap
securely into place.
You will hear several
clicks.
A
B
Step 2: Attach sensor to patch
• Ensure no gaps are
visible between the
blue cradle and sensor.
• To confirm recording,
a solid green light is
displayed. The green
light will blink for a
period of time and then
turn off.
No
Gap
Gap
Not seeing the green light sequence?
Contact Patient Services at 1-877-593-6421
or visit myheartmonitor.com.
C
Green
light
D
* Logo design on device in this guide may dier from the logo
on the actual device supplied in kit.
Getting started

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
7
• Locate the patch placement template in the
kit and follow its instructions for use.
• Remove clear backing from the patch.
C
D
Step 2: Apply ePatch
Getting started

8
Step 2: Apply ePatch, continued
• Apply the ePatch to your chest using the template as
a guide as shown. Use a mirror for guidance. Remove
template when finished.
• Press the patch firmly against your skin then remove
top white paper.
• Congratulations! You completed the set-up process.
Reference: This is how the
ePatch should be placed
inside the template cutout.
Step 1
Remove backing from
ePatch.
Step 2
Face mirror and put the
top of the ePatch
template at the base of
your neck. Then, place
ePatch on your chest.
Holter ePatch template
Base of neck
Step 3
Take the ePatch
ŊâęĻē½ŊâġŻŮġŏľØüâłŊ֤
and remove cover paper
from ePatch.
© 2022 Koninklijke Phili ps N.V. All rights reser ved. Doc 220- 0603-01 Rev. D
A
B
Getting started

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
9
• Whenever you feel a heart-related symptom, record the
symptom in the Holter symptom event diary included in the kit.
• Make sure to write that symptom and how you are feeling in
the diary.
• Locate the diary in your kit and fill in your name, address,
physician’s name, and the start date. After your monitoring
period has ended, fill in the date you removed ePatch.
• Do not forget to include the date and time of each symptom.
Record symptoms
Good to know

10
Changing the patch
Change the patch if it begins to loosen. Patches should
last approximately 5 days.
*
To change and apply a new patch:
• Remove the patch by pulling the clear adhesive away
from your body.
• Apply downward pressure on the tab to snap/break it
off. This will require some force.
* Depending on how long your service lasts, you will need to change your patch every 5 days or sooner.
Good to know

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
11
Changing the patch, continued
• Hold the sensor as shown and slide it forward to
remove it from the patch.
• Discard the used patch, NOT the sensor
Good to know

12
Changing the patch, continued
• Additional patches are provided in the kit. Refer to the
Getting Started section of this guide to set up and apply a
new patch.
• Note: If applying the patch in the same area, please do not
scrub the skin with the scrub pad before applying a new
patch.
• If skin is broken or irritated, select an area on your skin
in a slightly different location or position. Please refer to
the illustration.
Good to know

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
13
• Continue to wear ePatch for the duration prescribed
by your physician.
• Mild itching or irritation underneath the patch may occur and is
usually temporary. If more significant itching or irritation develops
or persists, contact Patient Services at 1-877-593-6421. They may
direct you to contact your physician.
• Record any events or symptoms you may feel in the Holter
symptom event diary (see directions listed previously in this guide).
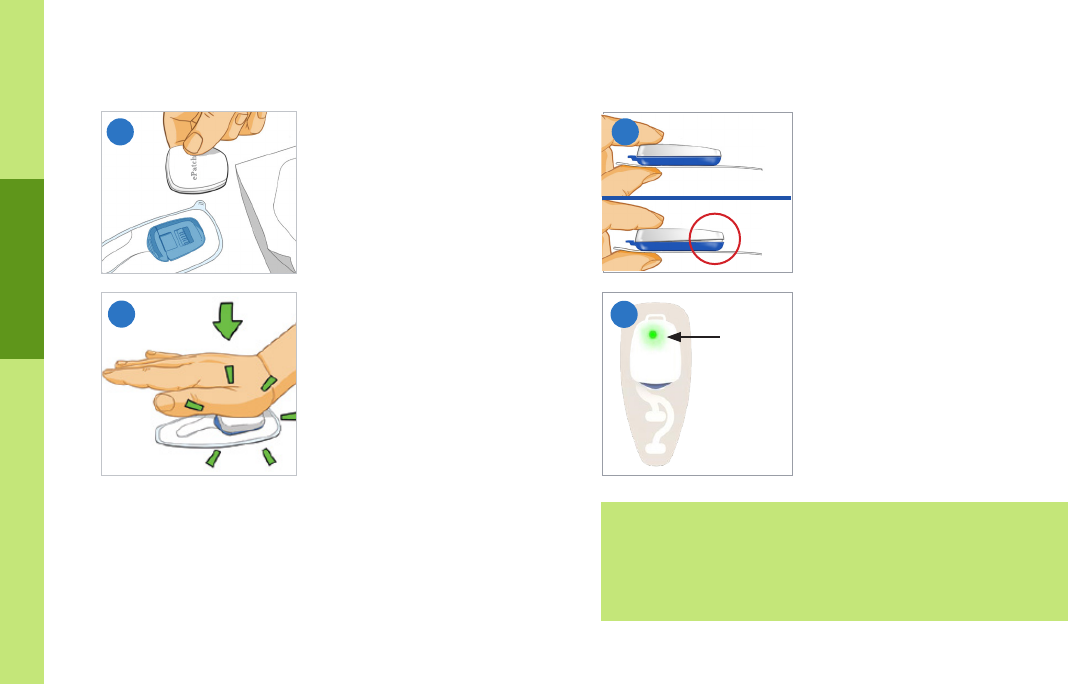
• When you insert the sensor into the patch, make sure that you:
– Press the sensor down onto the patch rmly and you may hear
several clicks
– Ensure no gaps are visible between the blue cradle and sensor.
– Conrm ePatch is recording (a solid green light is displayed,
then the green light will blink for a period of time and then
turns o). If this does not occur, please call Patient Services
(1-877-593-6421) or visit myheartmonitor.com.
Important information
Green
light
No
Gap
Gap
Good to know

14
Showering instructions
Shower or exercise as normal
while wearing ePatch.
Do not swim or bathe. ePatch is
water-resistant, not waterproof.
Good to know

For assistance, please call 1-877-593-6421 or visit myheartmonitor.com
15
Returning the equipment
Step 1
Important: Do not throw the box away. You will
need to use it to return the equipment and supplies.
Step 2
When you are ready to return ePatch, pack up the
sensor, unused patches, Holter symptom event diary
and any other supplies, and place into the kit.
Step 3
Remove the protective strip to expose the adhesive.
Seal the kit shut and then follow the return
instructions to ship the kit back to Philips. There is no
cost to you to mail back the equipment.
Good to know

16
Patient name:
1. You are being monitored using Philips extended Holter – ePatch, a prescription-only, continuously recording
and non-transmitting cardiac device that contains a rechargeable lithium-ion battery.
2. If you travel by aircraft while wearing the ePatch, please show TSA authorities this card
at the security checkpoint and let them know this cardiac monitoring device:
• Is attached to your skin and cannot be removed or else it will prematurely end your service.
• Does not transmit data wirelessly.
• Contains a lithium-ion battery that can be carried on to the plane per FAA regulations regarding
lithium-ion batteries.
*
If you are traveling outside the U.S. anytime during your monitoring period, contact Patient Services at
(1-877-593-6421) for assistance, Mondays–Fridays from 8am–8:30pm ET and Saturdays from 8am–4:30pm ET.
If you need assistance during your monitoring period, contact Patient Services at 1-877-593-6421
for assistance, Monday–Friday: 8am–8:30pm ET and Saturday–Sunday: 8am–4:30pm ET.
* For more information, visit https://www.faa.gov/
Travel information
Good to know

17

18
Indications for use
ePatch is indicated for use on patients who may be asymptomatic or who may suer from
transient symptoms such as palpitations, shortness of breath, dizziness, light headedness,
pre-syncope, syncope, fatigue, chest pain and/or anxiety.
ePatch is intended for use by adolescents 18-21 and adults.
Contraindications
The sensor is not intended for use on:
• Patients with implanted pacemakers.
• The compatible patch is not intended for use in the following cases:
• On patients with known allergies to adhesive materials or hydrogel.
• The compatible patch should not be placed on broken, damaged, or irritated skin.
Caution: Federal law restricts this device to sale by or on the order of a physician.
Precautions
Avoid contact with the eyes or mucus membranes of gels, alcohol, acetone, or any
substance used in the placement or removal of the patch. This can damage the eyes or
mucus membranes of the patient.
Do not use an obviously broken sensor. This can cause electric discharge or decrease the
quality of the acquired ECG signals.
Do not touch the terminals at the backside of the sensor or let them touch other
conductive parts or earth. This may damage the sensor.
Minimize the number of devices connected to the patient. Otherwise, there is a risk of
accumulation of leakage current.
Store and use the sensor within temperature, pressure, and humidity ranges specied
in the specications section. Avoid exposing any part of the sensor to heat sources,
heat radiators and replaces, direct exposure to sunlight, nebulizers, or electrical
steam kettles. Temperature changes cause condensation and moisture that can lead
to malfunction of the sensor. Before using the sensor, allow the sensor to acclimate
to ambient temperature. For reference, if the temperature dierence between the
sensor and the environment is above 10ºC, a 20 minutes wait time in an intermediate
temperature is recommended.
The sensor is NOT water resistant when worn with the patch; therefore, do not bathe or
swim while wearing the sensor in the patch set-up.
Do not expose the internal parts of the sensor to any liquids.
Mobile phones, transmitters, and similar equipment generating radio frequency (RF)
emissions should not be used or placed next to the sensor during recordings. This can
aect the to sensor. Follow the recommendations regarding the separation distance
specied in the manufacturer’s declaration for EMC in this Instruction Manual, see Annex
1 of the ePatch Sensor IFU.
Warnings
The sensor is not intended for use on infants, or on pregnant and/or
breastfeeding women.
The sensor is not intended for use on patients with implanted pacemakers.
Do not use the sensor in an X-ray, computed tomography (CT), or magnetic resonance
imaging (MRI) environment. This may aect the scanning results, may lead to
malfunction of the sensor, and it may injure the patient.
Remove sensor prior to debrillation.
Do not tamper with, disassemble or modify sensor or accessories, as this may aect
functionality or performance. A slight electrical sensation may be experienced.
Use the sensor with compatible accessories supplied by the manufacturer. Otherwise,
electrical shock or damage to the sensor may occur. In addition, the ECG signal quality
could be aected.
Use of other equipment or accessories not specied in this Instructions For Use document
might lead to skin irritations, allergy, electrical shock, and malfunction of the sensor. Use
of other chargers may damage the device and/or the accessories.
Keep products out of reach of infants, children and pets, as this could potentially cause
a choking hazard or cause suocation if placed over face or mouth. There is a danger of
strangulation if the provided USB Cable and/or lead wires are misused.
Please refer to the Instructions for Use associated with the compatible accessory for their
specic Warnings.
ePatch addendum to the patient education guide

19
Specications
ePatch sensor
Device classication
(EN 60601-1): Class
Internally Powered, Type BF applied parts, not protected
against debrillator, no functional earth terminal
Data acquisition 1, 2, or 3 channels ECG, Event Marker
a
Recording time Up to 14 days
(the recording time is congurable
b
by manufacturer)
Sampling rate 128, 256, 512, or 1024 Hz
a
Resolution 12 bit or 16 bit, depending on customer presence
Frequency response 0.05 to 55 Hz
Input range
ECG Channels
180 mVpv (Peak-to-Valley) CMRR
(common mode rejection ratio): >60 dB @ 50/60 Hz
Input impedance 10 MΩ
Connections 1 ePatch Specic 8-Terminals Connector
for connection to a compatible patch
Storage medium 2 GB internal storage
Maximum data le size 2 GB EFS-le (ePatch File System)
Expected service life Minimum 300 uses or two years (whichever occurs rst)
Battery (sensor)
The sensor is powered by an integrated battery with the following specications. The
battery is not replaceable.
Type Rechargeable lithium-ion polymer battery
Nominal capacity 500 mAh
Nominal voltage 3.7 V
Charging voltage 4.2 V
Battery life 300 charge cycles
Maximum charge
current
500 mA
Dimensions and weight (sensor)
Dimensions (W x H x D) 40 x 49 x 12 mm
Weight 20 g
Environmental conditions and device life (sensor)
Enclosure protection
degree
IP24 (when the sensor is rmly connected
to a compatible patch/electrode)
Operating conditions
Temperature +5°C to +40°C
Pressure 700 – 1060 hPa
Relative humidity 15% - 90 (non-condensation)
Transport and storage conditions (including between uses)
Temperature - 25°C to +50°C
Relative humidity 15% - 93% (non-condensation)
Device life Non-perishable, battery charge level to be maintained
Caution: Exceeding the recommended operating, storage, and transportation conditions
may result in reduction of the performance of the sensor and/or accessories.
Note: For environmental conditions for the compatible accessories, refer to the
instructions for use specic to the accessory.
a The number of recorded ECG channels and the sampling frequency depends on the conguration of your sensor. Note that not
all combinations of channels and sampling frequencies are possible.
b The default recording time for a conguration with two ECG channels and a sampling frequency of 256Hz is 14 days but other
congurations are possible, if requested from manufacturer.
The maximum possible recording time is increased when the number of recorded ECG channels and/or the sampling frequency is
decreased. Likewise, the maximum possible recording time is decreased when the number of recorded ECG channels and/or the
sampling frequency is increased. Note that the recording time of the sensor might be congured to be less than the maximum
possible recording time.

20
System requirements
The sensor requires a standard computer with the following minimum
specications to read out the recorded data:
• Microsoft® Windows 7 or Mac OS X 10.7 by Apple Inc.
• 1.5 GHz processor
• 512 MB RAM
• USB 2.0 port for connection of the sensor charge adapter USB cable
• 1 GB of free hard-drive space
Validated accessories
The sensor is used in combination with other medical accessories specied
by Braemar:
• ePatch LWA
• ePatch Flex Electrode
• BTP-1000P Patch
• ePatch -compatible ECG electrodes
• Sensor Charge Adapter
• The USB power adapter (USB 5.0 VDC, 500 mA)
Glossary of Symbols for ePatch sensor
Symbol
Description
2797
CE mark Indicates that the product conforms with standards
for products sold within the European Union
WEEE (Waste Electrical and Electronic Equipment) Directive
(2002/96/EC)
Type BF Applied Part
philips.com/IFU
Electronic instructions for use
Refer to instruction manual
Protect from moisture
Prescription use only (U.S. Federal Law)
Catalogue number
Manufacturer
Serial number
Year of Manufacture
European Authorized Representative
MR Unsafe
21
Please read this document carefully before activating
the monitor.
Activating monitoring service is your acceptance of the terms of this Agreement. If
you do not agree with the terms of this document please notify Customer Service at
1-866-426-4401 immediately.
Privacy and condentiality.
Activating monitoring service serves as your electronic signature indicating you
acknowledge that you have received a copy of BioTelemetry’s Notice of Condentiality
and Privacy Practices, which is incorporated in this agreement below. This
acknowledgment is required by the Health Insurance Portability and Accountability
Act (HIPAA) to ensure that you have been made aware of your privacy rights. You give
BioTelemetry your consent and permission to communicate with other members of your
household, if necessary, with regard to your BioTelemetry service. You also authorize
BioTelemetry to provide your monitoring data to your physician and his /her sta and
to Emergency Medical Services by phone, e-mail, fax or through secure Internet access.
You consent to receiving calls from BioTelemetry and its aliates or authorized agents
on your landline or cellular telephone related to the service or payment related to the
service. For example, BioTelemetry or its aliate or authorized agent may contact you in
order to obtain the loaned BioTelemetry Monitoring System (“System”) or seek payment
for the value of the System. You understand that such communications may include the
use of prerecorded voice messages and/or automatic telephone dialing systems.
Assignment of benets
I request that payment of authorized health insurance benets, including Medicare
benets, if I am a Medicare beneciary, to be made on my behalf to CardioNet, LLC. ( a
subsidiary of BioTelemetry, Inc.) for any medical services provided to me by CardioNet.
I authorize any holder of medical and/or insurance information about me to release
to CardioNet, my health insurance carrier, or the Centers for Medicare and Medicaid
Services (CMS) any information needed to determine these benets or the benets
payable for related services provided under this agreement. This assignment includes
all dates of services rendered by CardioNet for all insurance plans. A copy of this
authorization will be sent to CMS or my health insurance carrier if requested. The original
will be kept on le by CardioNet. I understand that I am fully responsible to CardioNet
for any co-payments, co-insurance, deductibles, payments made directly to me by my
health insurance carrier for CardioNet services, and, when allowed by law, services not-
covered or payable under my health insurance plan. I also understand that activating
monitoring services serves as my electronic signature, and that I am accepting nancial
responsibility as explained above for all payment for services received from CardioNet. By
signing this document and/or accepting these terms electronically, I acknowledge that
I have received a copy of CardioNet’s Notice of Privacy Practices. This acknowledgment
is required by the Health Insurance Portability and Accountability Act (HIPAA) to ensure
that I have been made aware of my privacy rights.
Service agreement
Financial Terms I understand that I am fully responsible and agree to pay for any co-
payments, co-insurance, deductibles, all payments made directly to me by my insurer for
CardioNet services, and when allowed by law, services not-covered (not payable) under
my health insurance plan. I acknowledge that I am nancially responsible for the loaned
System (sensor, monitor, and accessories), which I am obligated to return to CardioNet
upon completion of the service. If I do not immediately return the System, I hereby
authorize CardioNet to invoice me for, and agree to pay CardioNet, the value of the
Monitoring System and any associated collection costs should collection or legal costs be
incurred by CardioNet.
Operational notices
I hereby acknowledge that, given the variance in cellular phone coverage and signal
strength, the System may not always provide continuous transmission of my ECG rhythm
to the Monitoring Center. In the event that there is no cellular phone coverage or
adequate signal strength to transmit recorded events, I will move to an area to optimize
transmission capability or connect the monitor and base to a direct telephone line as
requested. I hereby acknowledge that the System is intended to aid in diagnosis only,
and is not designed for prevention or treatment of any event or condition. I agree
to immediately discontinue use of the System upon any sign of discomfort or other
problems directly related to the System, and to promptly report such discomfort or
other problems to BioTelemetry. I give BioTelemetry and its subsidiaries my consent and
permission to communicate with other members of my household, if necessary, with
regard to my BioTel Heart service. I also authorize BioTelemetry and its subsidiaries to
provide my monitoring data to my physician and his /her sta and to Emergency Medical
Services by phone, e-mail, fax or through secure Internet access.
CardioNet, LifeWatch, and BioTel Heart are trademarks of BioTelemetry, Inc.
Terms and conditions of the BioTelemetry service agreement
22
This notice describes how medical information about you may be used and disclosed
and how you can get access to this information. Please review it carefully.
Uses and disclosures of your health information
Research: Under certain circumstances, we may disclose your health information to
researchers when their research has been approved by an institutional review board
or privacy board that has reviewed the research proposal and protocols to ensure the
privacy of your health information.
Death; Organ Donation: We may disclose your health information to a coroner, medical
examiner, funeral director or organ procurement organization for certain purposes as
necessary for each to carry out their duties. For example, if you are an organ donor, we
may disclose your health information to an organ procurement organization as necessary
to facilitate organ donation or transplantation. We may disclose your health information
to a coroner or medical examiner to identify a deceased person or determine the cause
of death.
Public Health and Safety: We may disclose your health information in connection with
certain public health reporting activities. For example, we may disclose your health
information to a public health authority authorized to collect or receive such information
such as state health departments and federal health agencies. We may use and disclose
your health information to the extent necessary to avert a serious and imminent threat
to your health or safety or the health or safety of others. We may disclose your health
information to appropriate authorities if we reasonably believe that you are a possible
victim of abuse, neglect, domestic violence or other crimes. We may also disclose your
health information to the Food and Drug Administration (FDA) or a person subject to
the jurisdiction of the FDA for the purpose of activities related to the quality, safety or
eectiveness of an FDA-regulated product or activity.
Required by Law: We will use or disclose your health information when we are required
to do so by law.
Process and Proceedings: We may disclose your health information in response to a
court or administrative order, subpoena, discovery request or other lawful process.
Law Enforcement: We may disclose your health information, so long as applicable legal
requirements are met, to a law enforcement ocial, such as for providing information to
the police about the victim of a crime.
Inmates: We may disclose your health information if you are an inmate of a correctional
institution and we created or received your health information in the course of providing
care to you.
Military and National Security: We may disclose your health information to military
authorities if you are a member of the Armed Forces. We may disclose your health
information to authorized federal ocials for lawful intelligence, counterintelligence,
protection of the President and authorized persons or foreign heads of state and other
national security activities.
Workers’ Compensation: We may disclose your health information as authorized by and
to the extent necessary to comply with laws relating to workers’ compensation or other
similar programs, established by law, that provide benets for work-related injuries or
illness without regard to fault.
Health Oversight: We may disclose your health information in connection with certain
health oversight activities of licensing and other agencies, such as audit, investigation,
inspection, licensure, or disciplinary actions, and civil, criminal, or administrative
proceedings.
Required by the Secretary of Health and Human Services: We may be required to
disclose your health information to the Secretary of the United States Department of
Health and Human Services to investigate or determine our compliance with certain legal
requirements.
National Instant Criminal Background Check System: We may use or disclose
your health information for purposes of reporting to the National Instant Criminal
Background Check System the identity of an individual who is prohibited from possessing
a rearm under 18 U.S.C. 922(g)(4).
Business Associates: We may disclose your health information to persons who perform
functions, activities or services to us or on our behalf that require the use or disclosure
of your health information. To protect your health information, we require the business
associate to appropriately safeguard your information.
To you: We will disclose your health information to you, as described in the Individual
Rights section of this notice.
Notice of confidentiality and privacy practices
23
Uses and disclosures that may be made either with your
agreement or the opportunity to object
Unless you object, we may disclose to a member of your family, a relative, a close friend or
any other person you identify, orally or in writing, your health information that directly
relates to that person’s involvement in your health care. If you are unable to agree or
object to such disclosure, we may disclose such information as necessary if we determine
that it is in your best interest based on our professional judgment. We may use or disclose
your health information to notify or assist in notifying a family member, personal
representative or any other person that is responsible for your care of your location or
general condition.
Uses and disclosures based on your written authorization
Marketing: We must obtain your written authorization to use and disclose your health
information for most marketing purposes.
Sale of health information: We must obtain your written authorization for any
disclosure of your health information which constitutes a sale of health information.
Other uses: Other uses and disclosures of your health information will be made only
with your written authorization, except as described in this notice or as otherwise
required or allowed by applicable law. In the event that we ask for your authorization
to use or disclose your health information, we will provide you with an appropriate
authorization form. Once you’ve given us a written authorization, you can revoke that
authorization at any time, except to the extent that we have taken action in reliance on
your authorization.
Individual rights
Access: You have the right to see or get an electronic or paper copy of your health
information by submitting a request to us in writing using the information listed at the
end of this notice. There are certain exceptions to your right to obtain a copy of your
health information. For example, we may deny your request if we believe the disclosure
will endanger your life or that of another person. Depending on the circumstances of the
denial, you may have a right to have this decision reviewed. We will charge you a fee to
cover the costs incurred by us in complying with your request.
Disclosure accounting: You have the right to an accounting of disclosures of your
health information made by us by submitting a request to us in writing using the
information listed at the end of this notice. This right only applies to instances when
we or our business associates disclosed your health information for purposes other
than treatment, payment, health care operations, upon your written authorization,
and certain other activities. The right to receive this information is subject to certain
exceptions, restrictions and limitations. You must specify a time period, which may not be
longer than 6 years. You may request a shorter timeframe. You have the right to one free
request within any 12-month period, but we may charge you for any additional requests
in the same 12-month period. We will notify you about any such charges, and you are free
to withdraw or modify your request in writing before any charges are incurred.
Restriction requests: You have the right to request restrictions on the use and disclosure
of your health information by submitting a request to us in writing using the information
listed at the end of this notice. Your request must state the specic restriction requested
and to whom you want the restriction to apply. We are not required to agree to these
additional restrictions, except we must agree not to disclose your health information
to your health plan if the disclosure (1) is for payment or health care operations and is
not otherwise required by law, and (2) relates to a health care item or service which you
paid for in full out of pocket. If we agree to a restriction, we will abide by our agreement
(except in an emergency).
Condential communication: You have the right to receive certain communications
condentially. That means you can request that we communicate with you by alternative
means or to an alternative location by submitting a request to us in writing using the
information listed at the end of this notice. We will accommodate your request if it is
reasonable and species the alternative means or location. We may also condition this
accommodation by asking you for information as to how payment will be handled.
Amendment: You have the right to amend your health information in our records for
as long as we maintain the information. You must make a request in writing, using the
24
information listed at the end of this notice, to obtain an amendment. Your written
request must explain why the information should be amended. If we agree to amend
your health information, we will make reasonable eorts to inform others of the
amendment and to include the changes in any future disclosures of that information.
We may deny your request if, for example, we determine that your health information is
accurate and complete. If we deny your request, we will send you a written explanation
and allow you to submit a written statement of disagreement to be appended to the
information you want amended.
Paper notice: If you receive this notice electronically you are entitled to receive this
notice in written form. Please contact us using the information listed at the end of this
notice to obtain this notice in written form.
Breach: You have the right to be notied if you are aected by a breach of unsecured
health information.
Questions and complaints
If you want more information about our privacy practices or have questions or concerns,
please contact us using the information listed at the end of this notice.
If you are concerned that we may have violated your privacy rights, or you disagree with
a decision we made about your rights to your health information, you may submit a
complaint to us using the information listed at the end of this notice. You may also submit
a complaint to the U.S. Department of Health and Human Services.
We support your right to protect the privacy of your health information. We will not
retaliate against you in any way if you choose to le a complaint with us or with the U.S.
Department of Health and Human Services.
Contact information
BioTelemetry, Inc.
Privacy Ocer
1000 Cedar Hollow Road, Suite 102
Malvern, PA 19355
Telephone: 610.729.7000
email: [email protected]
Update Eective Date: September 3, 2020
I certify that I understand and agree to the foregoing terms
and to the following standard terms and conditions.
1. Use of Cardiac Monitoring System (“System”) and access to and use of Monitoring
Service (“Service”). Subject to Patient’s compliance with the terms and conditions
indicated within this Patient Education Guide (the “Agreement”), BioTelemetry hereby
grants Patient a personal, nonexclusive, nontransferable license to use the System and to
access and use the features and functions of the Service solely for purposes of monitoring
Patient’s heart rate as prescribed by Patient’s physician. Patient expressly acknowledges
and agrees that the Service, which is available only by physician prescription, is used
solely to assist physicians in diagnosis and treatment, and is not intended for use as an
emergency response system for patients who may experience serious or life-threatening
medical problems. Patient is aware that cell phone coverage limitations and delays in
land-line telephone communications could signicantly delay transmission and analysis
of patient monitoring data. Patient agrees to contact BioTelemetry immediately if
problems are experienced using the system or if signs of physical discomfort occur,
and to discontinue use of the system if the physician or BioTelemetry believe service
discontinuation is advisable. Patient shall not, in whole or in part, sublicense, provide
access to, tamper with, modify, distribute, use in a service bureau or time-sharing
capacity, export in violation of applicable laws and regulations, rent, loan, transfer,
disassemble, or reverse engineer or create a derivative work of the System or Service.
Patient shall not, in whole or in part, transfer or assign this Agreement or any right
granted hereunder, except upon the prior written consent of BioTelemetry. Any
prohibited transfer or assignment shall be null and void. Subject to the licenses granted
herein, as between BioTelemetry and Patient, BioTelemetry holds all right, title and
interest in and to the System and the Service including, without limitation, any patents,
trademarks, trade secrets, copyrights or other intellectual property rights therein.
BioTelemetry reserves all rights not expressly granted to Patient under this Agreement.
2. Term and Termination. This Agreement shall commence on the date that BioTelemetry
accepts Patient’s enrollment hereunder, and shall continue until terminated by
either party as set forth herein. Either party may terminate this Agreement, for any
or no reason, upon thirty (30) days’ written notice to the other party, except that this
Agreement shall immediately terminate if Patient breaches Paragraph 1 above. Upon
any termination of this Agreement, Patient shall immediately discontinue all use of
the Service, and shall promptly return the System to BioTelemetry. The limitations in
Paragraph 1, and Paragraphs 3-6 shall survive any termination of this Agreement.
3. NO WARRANTY. THE SYSTEM AND THE SERVICE ARE PROVIDED BY BIOTELEMETRY
HEREUNDER SOLELY ON AN “AS-IS” AND “AS AVAILABLE” BASIS WITHOUT WARRANTY
OF ANY KIND. TO THE MAXIMUM EXTENT PERMITTED UNDER APPLICABLE LAW,
BIOTELEMETRY HEREBY DISCLAIMS ANY AND ALL WARRANTIES, EXPRESS, IMPLIED OR
STATUTORY, INCLUDING, BUT NOT LIMITED TO, ANY WARRANTY OF MERCHANTABILITY,
25
FITNESS FOR A PARTICULAR PURPOSE, TITLE, NON-INFRINGEMENT AND/OR QUIET
ENJOYMENT, AS WELL AS ANY IMPLIED WARRANTIES OTHERWISE ARISING OUT OF
COURSE OF DEALING, COURSE OF PERFORMANCE OR TRADE USAGE. PATIENT FURTHER
ACKNOWLEDGES AND AGREES THAT BIOTELEMETRY SHALL NEITHER BE RESPONSIBLE
NOR LIABLE FOR PATIENT’S INABILITY TO ACCESS OR USE THE SERVICE AS A RESULT OF
ANY DEFICIENCY IN THE INTERNET, THE TELEPHONE SERVICE, OR OTHER CONNECTION
BETWEEN BIOTELEMETRY AND PATIENT. PATIENT EXPRESSLY ACKNOWLEDGES AND
AGREES THAT NEITHER THE SYSTEM, NOR THE SERVICE (AS WELL AS ANY SUPPORT
GIVEN BY ANY BIOTELEMETRY SUPPORT STAFF), NOR ANY MATERIAL AVAILABLE
THROUGH PATIENT’S USE OF THE SYSTEM OR SERVICE IS INTENDED TO PROVIDE
PATIENT WITH MEDICAL ADVICE, A DIAGNOSIS OR TREATMENT. PATIENT MUST ALWAYS
SEEK THE ADVICE OF PATIENT’S PHYSICIAN OR OF ANOTHER QUALIFIED MEDICAL
PRACTITIONER WITH ANY QUESTIONS PATIENT MAY HAVE REGARDING A SPECIFIC
MEDICAL CONDITION OR PERCEIVED CONDITION.
4. LIMITATION OF LIABILITY. TO THE MAXIMUM EXTENT PERMITTED UNDER APPLICABLE
LAW: (I) IN NO EVENT SHALL BIOTELEMETRY OR ITS SUBSIDIARIES, AFFILIATES, OFFICERS,
DIRECTORS, EMPLOYEES AND AGENTS, ITS LICENSORS OR SUPPLIERS BE LIABLE TO
PATIENT FOR ANY INDIRECT, INCIDENTAL, SPECIAL, CONSEQUENTIAL OR PUNITIVE
DAMAGES ARISING OUT OF OR RELATED TO THIS AGREEMENT INCLUDING, WITHOUT
LIMITATION, LOST PROFITS, COSTS OF DELAY, ANY FAILURE OF DELIVERY, BUSINESS
INTERRUPTION, COSTS OF LOST OR DAMAGED DATA, UNAUTHORIZED DISCLOSURE TO
OR ACCESS OF PATIENT DATA, OR LIABILITIES TO THIRD PARTIES ARISING FROM ANY
PERSONAL INJURY OR PROPERTY DAMAGE CLAIM OR ANY OTHER TYPE OF CLAIM, EVEN
IF BIOTELEMETRY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES; AND, (II)
IN NO EVENT SHALL BIOTELEMETRY’S AGGREGATE LIABILITY UNDER THIS AGREEMENT
EXCEED THE AMOUNT PAID BY PATIENT TO BIOTELEMETRY UNDER THIS AGREEMENT.
THE PARTIES AGREE THAT THE ALLOCATION OF LIABILITY SET FORTH IN THIS SECTION 5
FORMS AN ESSENTIAL BASIS OF BIOTELEMETRY’S WILLINGNESS TO GRANT PATIENT THE
USE OF THE SYSTEM AND ACCESS TO AND USE OF THE SERVICE AND IS INDEPENDENT OF
EACH AND EVERY LIMITED REMEDY THAT PATIENT MAY HAVE.
5. Indemnity. Patient agrees to indemnify and hold harmless BioTelemetry, Inc., it’s
subsidiaries, and its ocers, directors, employees, agents and suppliers from and against
all claims of third parties arising out of or related to Patient’s use or misuse of the System
and/or the Service, or attributable to Patient’s breach of this Agreement. BioTelemetry
shall control the defense and any settlement of such claim, and Patient shall cooperate
with BioTelemetry in defending against such claims.
6. General Provisions. This Agreement may be modied or amended only by a written
instrument signed by Patient and BioTelemetry. Any terms and conditions issued by
Patient shall not be binding on BioTelemetry, Inc., or it’s subsidiaries, ocers, directors,
employees, agents or suppliers, and shall not modify these Terms and Conditions. No
term or provision contained herein shall be deemed waived and no breach excused
unless such waiver or consent shall be in writing and signed by the party against whom
enforcement thereof is sought. Neither party hereto shall be liable to the other for
any failure to perform its obligations under this Agreement due to causes beyond the
reasonable control of that party, including, but not limited to, strikes, boycotts, labor
disputes, embargoes, unavailability of or failures due to telecommunication networks
(including, without limitation, the Internet), acts of God, unavailability of or insucient
utilities, acts of public enemy, acts of governmental authority, oods, riots, or rebellion.
This Agreement shall be governed by and construed solely in accordance with the laws
of the State of Pennsylvania, without reference to its choice of law rules. Any and all
proceedings arising under or in any way relating to this Agreement shall be maintained
in the state or federal courts located in Chester County, Pennsylvania, which courts
shall have exclusive jurisdiction for such purpose, and Patient hereby consents to the
personal jurisdiction of such courts. Patient acknowledges that in the event of an actual
or threatened violation of the terms and conditions of this Agreement, BioTelemetry may
not have an adequate monetary remedy and shall be entitled to seek injunctive relief
without any requirement to post bond, in addition to any other available remedies. If
any term or provision of this Agreement is illegal or unenforceable, it shall be deemed
adjusted to the minimum extent to cure such invalidity or unenforceability and all other
terms and provisions of this Agreement shall remain in full force and eect.
26
Patients’ rights and responsibilities
This document describes a summary of the rights and responsibilities of the patient using
Philips’ ECG diagnostic services. This document may not be comprehensive: please refer
to the labeling or your clinical provider for further information or questions.
Rights:
• The patient has the right, within the law, to personal and information privacy, as
manifested by the right to:
– Make recording and submit transmissions in surroundings designed to
assure privacy.
– Expect that any discussion involving the patient will be conducted discreetly.
– Medicare records will only be read by individuals directly involved in the patient’s
care or the monitoring of its quality.
– Expect that all communications and other records pertaining to care, including the
source of payment of treatment be treated as confidential.
– Expect appropriate services without discrimination based upon race, color, religion,
sex, handicap, sexual preference, or national origin.
– Expect that their psychosocial, spiritual, and cultural values are respected and
that they may express their spiritual beliefs and cultural practices that do not
harm others.
• The patient has the right to expect safety in so far as Philips practices and environment
are concerned.
• The patient has the right to obtain complete and current information concerning his
care from his/her physician. Philips provides a service to the patient and the physician.
It is the responsibility of the physician to discuss diagnosis, treatment & any known
prognosis. Please understand that Philips is not legally able to disclose the results of the
recordings.
• The patient has the right to expect a well-trained staff knowledgeable in areas related
to medical procedure and equipment used in these procedures. The staff is trained and
skilled in taking into consideration individual beliefs and values.
• All patients complaints will be addressed in accordance with the existing complaints/
grievance procedure.
• Patients under legal age have the right to have a surrogate decision maker properly
informed and educated.
• The patient has the right to access the company’s policy regarding charges and
payment responsibilities.
• The patient has the right to access the company’s Notice of Privacy Practices.
Responsibilities:
• The patient is responsible for making it known whether they clearly comprehend the
service and what is expected of them.
• The patient is responsible for following the instructions provided by Philips technicians.
• The patient is responsible for their actions if they refuse to use the service as prescribed
by physician.
• The patient is responsible to ask for explanations if you do not understand how the
service works or how to use the device.
• The patient is responsible to advise Philips of any dissatisfaction you may have
regarding the service.
• The patient is responsible to be considerate of the rights of Philips personnel.
• The patient is responsible to assure that the financial obligations associated with the
service are fulfilled.
• The patient is responsible for returning the assigned equipment to Philips, in proper
working order, upon completion of the service.
If you have any questions about your monitoring service or billing, please contact
Patient Services:
1-877-593-6421 (toll-free) or customerser[email protected]
Hours: Mon-Fri 8 am - 8:30 pm ET; Sat-Sun 8 am - 4:30 pm ET
Important reminder:
This device provides a diagnostic test. It is not an emergency response service. If at any
time you experience a symptom that you feel indicates a medical emergency, you should
immediately dial 911 for medical assistance.
27

© 2023 Koninklijke Philips N.V. All rights reserved. Doc 220-0690-01 Rev. G
Important reminder:
This device provides a diagnostic test. It is not an emergency response service. If at any time you experience a symptom
that you feel is a medical emergency, you should immediately dial 911 for medical assistance.
1000 Cedar Hollow Road, Suite 102, Malvern, PA 19355
