
Guidelines from the National Asthma Education
and Prevention Program
The goal of this asthma care quick
reference guide is to help clinicians
provide quality care to people who
have asthma.
Quality asthma care involves not only initial diagnosis and
treatment to achieve asthma control, but also long-term,
regular follow-up care to maintain control.
Asthma control focuses on two domains: (1) reducing
impairment—the frequency and intensity of symptoms and
functional limitations currently or recently experienced by a
patient; and (2) reducing risk—the likelihood of future asthma
attacks, progressive decline in lung function (or, for children,
reduced lung growth), or medication side effects.
Achieving and maintaining asthma control requires providing
appropriate medication, addressing environmental factors
that cause worsening symptoms, helping patients learn self-
management skills, and monitoring over the long term to
assess control and adjust therapy accordingly.
The diagram (right) illustrates the steps involved in providing
quality asthma care.
INITIAL VISIT
Diagnose asthma
Schedule follow-up appointment
Develop written asthma action plan
Initiate medication & demonstrate use
Assess asthma severity
Assess & monitor
asthma control
Schedule next
follow-up
appointment
Review asthma
action plan, revise
as needed
Maintain, step
up, or step down
medication
Review medication
technique &
adherence; assess
side effects; review
environmental control
FOLLOW-UP VISITS
EXPERT PANEL REPORT 3
This guide summarizes recommendations developed by the
National Asthma Education and Prevention Program’s expert panel
after conducting a systematic review of the scientific literature on
asthma care. See www.nhlbi.nih.gov/guidelines/asthma for the full
report and references. Medications and dosages were updated in
September 2011 for the purposes of this quick reference guide to
reflect currently available asthma medications.
Asthma Care
Quick Reference
DIAGNOSING AND MANAGING ASTHMA

KEY CLINICAL ACTIVITIES FOR QUALITY ASTHMA CARE
(See complete table in Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma [EPR-3])
Clinical Issue Key Clinical Activities and Action Steps
ASTHMA DIAGNOSIS
Establish asthma diagnosis.
Determine that symptoms of recurrent airway obstruction are present, based on history
and exam.
• History of cough, recurrent wheezing, recurrent difficulty breathing, recurrent
chest tightness
• Symptoms occur or worsen at night or with exercise, viral infection, exposure to allergens
and irritants, changes in weather, hard laughing or crying, stress, or other factors
In all patients ≥5 years of age, use spirometry to determine that airway obstruction is at
least partially reversible.
Consider other causes of obstruction.
LONG-TERM ASTHMA MANAGEMENT
GOAL:
Asthma Control
Reduce Impairment
Prevent chronic symptoms.
Require infrequent use of short-acting beta
2
-agonist (SABA).
Maintain (near) normal lung function and normal activity levels.
Reduce Risk
Prevent exacerbations.
Minimize need for emergency care, hospitalization.
Prevent loss of lung function (or, for children, prevent reduced lung growth).
Minimize adverse effects of therapy.
Assessment
and Monitoring
INITIAL VISIT: Assess asthma severity to initiate treatment (see page 5).
FOLLOW-UP VISITS: Assess asthma control to determine if therapy should be adjusted
(see page 6).
Assess at each visit: asthma control, proper medication technique, written asthma action
plan, patient adherence, patient concerns.
Obtain lung function measures by spirometry at least every 1–2 years; more frequently for
asthma that is not well controlled.
Determine if therapy should be adjusted: Maintain treatment; step up, if needed; step
down, if possible.
Schedule follow-up care.
Asthma is highly variable over time. See patients:
• Every 2–6 weeks while gaining control
• Every 1–6 months to monitor control
• Every 3 months if step down in therapy is anticipated
Use of
Medications
Select medication and delivery devices that meet patient’s needs and circumstances.
Use stepwise approach to identify appropriate treatment options (see page 7).
Inhaled corticosteroids (ICSs) are the most effective long-term control therapy.
When choosing treatment, consider domain of relevance to the patient (risk, impairment,
or both), patient’s history of response to the medication, and willingness and ability to use
the medication.
Review medications, technique, and adherence at each follow-up visit.
2
Asthma Care Quick Reference
KEY CLINICAL ACTIVITIES FOR QUALITY ASTHMA CARE (continued)
Clinical Issue Key Clinical Activities and Action Steps
Patient
Education for
Self-Management
Teach patients how to manage their asthma.
Teach and reinforce at each visit:
• Self-monitoring to assess level of asthma control and recognize signs of worsening
asthma (either symptom or peak flow monitoring)
• Taking medication correctly (inhaler technique, use of devices, understanding
difference between long-term control and quick-relief medications)
- Long-term control medications (such as inhaled corticosteroids, which reduce
inflammation) prevent symptoms. Should be taken daily; will not give quick relief.
- Quick-relief medications (short-acting beta
2
-agonists or SABAs) relax airway
muscles to provide fast relief of symptoms. Will not provide long-term asthma
control. If used >2 days/week (except as needed for exercise-induced asthma),
the patient may need to start or increase long-term control medications.
• Avoiding environmental factors that worsen asthma
Develop a written asthma action plan in partnership with patient/family (sample plan
available at www.nhlbi.nih.gov/health/public/lung/asthma/asthma_actplan.pdf).
Agree on treatment goals.
Teach patients how to use the asthma action plan to:
• Take daily actions to control asthma
• Adjust medications in response to worsening asthma
• Seek medical care as appropriate
Encourage adherence to the asthma action plan.
• Choose treatment that achieves outcomes and addresses preferences important to
the patient/family.
• Review at each visit any success in achieving control, any concerns about treatment,
any difficulties following the plan, and any possible actions to improve adherence.
• Provide encouragement and praise, which builds patient confidence. Encourage family
involvement to provide support.
Integrate education into all points of care involving interactions with patients.
Include members of all health care disciplines (e.g., physicians, pharmacists, nurses, respiratory
therapists, and asthma educators) in providing and reinforcing education at all points of care.
Control of
Environmental
Factors and
Comorbid
Conditions
Recommend ways to control exposures to allergens, irritants, and pollutants that make
asthma worse.
Determine exposures, history of symptoms after exposures, and sensitivities.
(In patients with persistent asthma, use skin or in vitro testing to assess sensitivity to
perennial indoor allergens to which the patient is exposed.)
• Recommend multifaceted approaches to control exposures to which the patient is
sensitive; single steps alone are generally ineffective.
• Advise all asthma patients and all pregnant women to avoid exposure to tobacco smoke.
• Consider allergen immunotherapy by trained personnel for patients with persistent
asthma when there is a clear connection between symptoms and exposure to an
allergen to which the patient is sensitive.
Treat comorbid conditions.
Consider allergic bronchopulmonary aspergillosis, gastroesophageal reflux, obesity,
obstructive sleep apnea, rhinitis and sinusitis, and stress or depression. Treatment of
these conditions may improve asthma control.
Consider inactivated flu vaccine for all patients >6 months of age.
3
Asthma Care Quick Reference

ASTHMA CARE FOR SPECIAL CIRCUMSTANCES
Clinical Issue Key Clinical Activities and Action Steps
Exercise-Induced
Bronchospasm
Prevent EIB.*
Physical activity should be encouraged. For most patients, EIB should not limit
participation in any activity they choose.
Teach patients to take treatment before exercise. SABAs* will prevent EIB in most patients;
LTRAs,* cromolyn, or LABAs* also are protective. Frequent or chronic use of LABA to
prevent EIB is discouraged, as it may disguise poorly controlled persistent asthma.
Consider long-term control medication. EIB often is a marker of inadequate asthma control
and responds well to regular anti-inflammatory therapy.
Encourage a warm-up period or mask or scarf over the mouth for cold-induced EIB.
Pregnancy
Maintain asthma control through pregnancy.
Check asthma control at all prenatal visits. Asthma can worsen or improve during
pregnancy; adjust medications as needed.
Treating asthma with medications is safer for the mother and fetus than having poorly
controlled asthma. Maintaining lung function is important to ensure oxygen supply to the fetus.
ICSs* are the preferred long-term control medication.
Remind patients to avoid exposure to tobacco smoke.
MANAGING EXACERBATIONS
Clinical Issue Key Clinical Activities and Action Steps
Home Care
Develop a written asthma action plan (see Patient Education for Self-Management, page 3).
Teach patients how to:
Recognize early signs, symptoms, and PEF* measures that indicate worsening asthma.
Adjust medications (increase SABA* and, in some cases, add oral systemic corticosteroids)
and remove or withdraw from environmental factors contributing to the exacerbation.
Monitor response.
Seek medical care if there is serious deterioration or lack of response to treatment.
Give specific instructions on who and when to call.
Urgent or
Emergency Care
Assess severity by lung function measures (for ages ≥5 years), physical examination, and
signs and symptoms.
Treat to relieve hypoxemia and airflow obstruction; reduce airway inflammation.
Use supplemental oxygen as appropriate to correct hypoxemia.
Treat with repetitive or continuous SABA,* with the addition of inhaled ipratropium
bromide in severe exacerbations.
Give oral systemic corticosteroids in moderate or severe exacerbations or for patients who
fail to respond promptly and completely to SABA.
Consider adjunctive treatments, such as intravenous magnesium sulfate or heliox, in severe
exacerbations unresponsive to treatment.
Monitor response with repeat assessment of lung function measures, physical
examination, and signs and symptoms, and, in emergency department, pulse oximetry.
Discharge with medication and patient education:
Medications: SABA, oral systemic corticosteroids; consider starting ICS*
Referral to follow-up care
Asthma discharge plan
Review of inhaler technique and, whenever possible, environmental control measures
*
Abbreviations: EIB, exercise-induced bronchospasm; ICS, inhaled corticosteroid; LABA, long-acting beta
2
-agonist; LTRA, leukotriene receptor
antagonist; PEF, peak expiratory flow; SABA, short-acting beta
2
-agonist.
4
Asthma Care Quick Reference
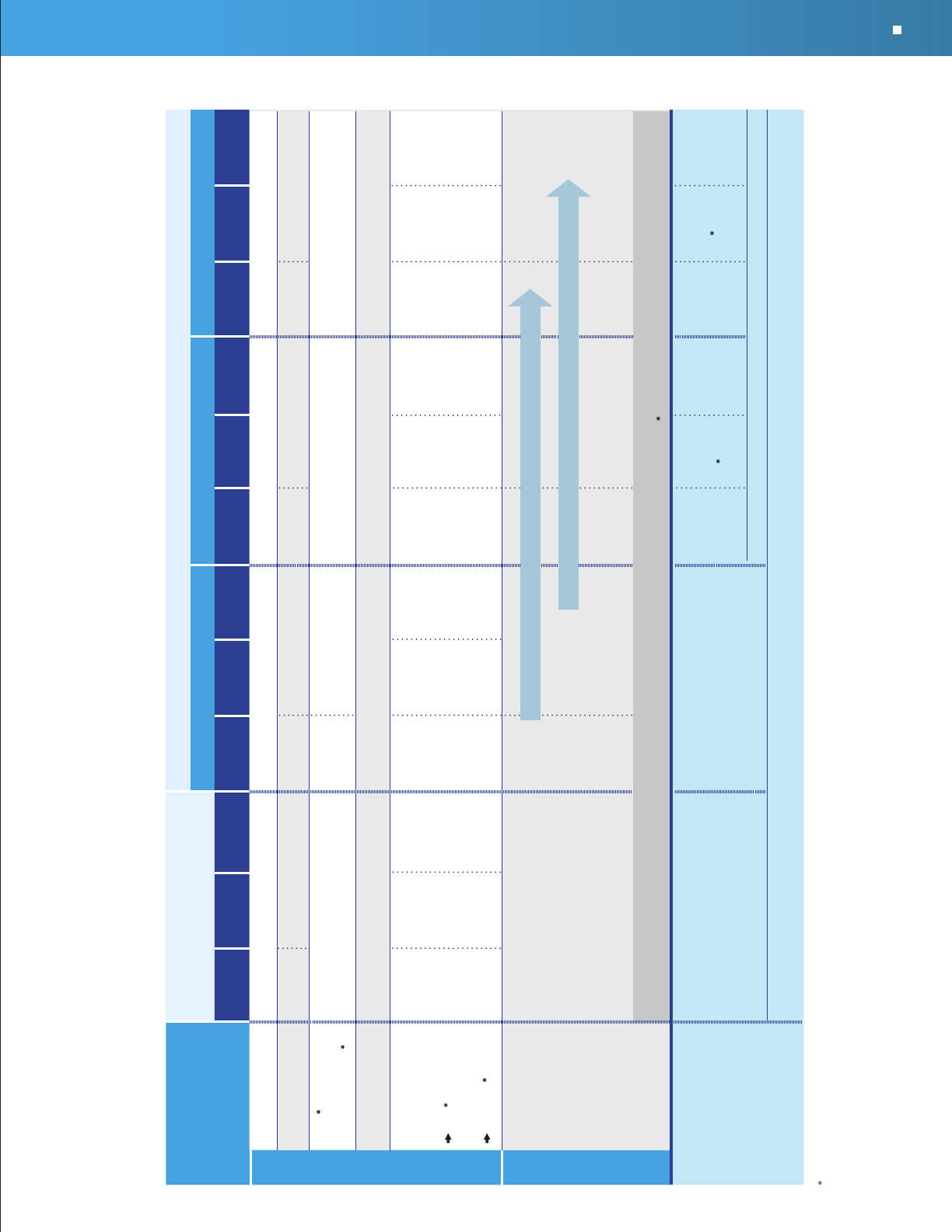
INITIAL VISIT: CLASSIFYING ASTHMA SEVERITY AND INITIATING THERAPY
(in patients who are not currently taking long-term control medications)
Level of severity (Columns 2–5) is determined by events listed in Column 1 for both impairment (frequency and intensity of symptoms and functional limitations) and risk (of
exacerbations). Assess impairment by patient’s or caregiver’s recall of events during the previous 2–4 weeks; assess risk over the last year. Recommendations for initiating therapy
based on level of severity are presented in the last row.
Components of
Severity
Intermittent
Persistent
Mild Moderate Severe
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Impairment
Symptoms
≤2 days/week >2 days/week but not daily Daily Throughout the day
Nighttime awakenings
0 ≤2x/month 1–2x/month 3–4x/month 3–4x/month >1x/week but not nightly >1x/week Often 7x/week
SABA use for
symptom control
(not to prevent EIB )
≤2 days/week
>2 days/week
but not daily
>2 days/week but
not daily and not more
than once on any day
Daily Several times per day
Interference with
normal activity
None Minor limitation Some limitation Extremely limited
Lung function
FEV
1
(% predicted)
FEV
1
/FVC
Not
applicable
Normal FEV
1
between
exacerbations
>80%
>85%
Normal FEV
1
between
exacerbations
>80%
Normal
†
Not
applicable
>80%
>80%
>80%
Normal
†
Not
applicable
60–80%
75–80%
60–80%
Reduced 5%
†
Not
applicable
<60%
<75%
<60%
Reduced >5%
†
Risk
Asthma exacerbations
requiring oral systemic
corticosteroids
‡
0–1/year
≥2 exacerb.
in 6 months,
or wheezing
≥4x per
year lasting
>1 day
AND risk
factors for
persistent
asthma
≥2/year
Consider severity and interval since last asthma exacerbation. Frequency and severity may fluctuate over time for patients in any severity category.
Relative annual risk of exacerbations may be related to FEV
1
.
Recommended Step for
Initiating Therapy
(See “Stepwise Approach for
Managing Asthma Long Term,”
page 7)
The stepwise approach is meant
to help, not replace, the clinical
decisionmaking needed to meet
individual patient needs.
Step 1 Step 2
Step 3
Step 3
medium-dose
ICS option
Step 3 Step 3
Step 3
medium-dose
ICS option
or Step 4
Step 4
or 5
Consider short course of oral systemic corticosteroids.
In 2–6 weeks, depending on severity, assess level of asthma control achieved and adjust therapy as needed.
For children 0–4 years old, if no clear benefit is observed in 4–6 weeks, consider adjusting therapy or alternate diagnoses.
Abbreviations: EIB, exercise-induced bronchospam; FEV
1
, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; SABA, short-acting beta
2
-agonist.
†
Normal FEV
1
/FVC by age: 8–19 years, 85%; 20–39 years, 80%; 40–59 years, 75%; 60–80 years, 70%.
‡
Data are insufficient to link frequencies of exacerbations with different levels of asthma severity. Generally, more frequent and intense exacerbations (e.g., requiring urgent care, hospital or intensive care admission, and/or oral corticosteroids)
indicate greater underlying disease severity. For treatment purposes, patients with ≥2 exacerbations may be considered to have persistent asthma, even in the absence of impairment levels consistent with persistent asthma.
Generally, more frequent and intense events indicate greater severity.
Generally, more frequent and intense events indicate greater severity.
5
Asthma Care Quick Reference

FOLLOW-UP VISITS: ASSESSING ASTHMA CONTROL AND ADJUSTING THERAPY
Level of control (Columns 2–4) is based on the most severe component of impairment (symptoms and functional limitations) or risk (exacerbations). Assess impairment by patient’s or caregiver’s
recall of events listed in Column 1 during the previous 2–4 weeks and by spirometry and/or peak flow measures. Symptom assessment for longer periods should reflect a global assessment,
such as inquiring whether the patient’s asthma is better or worse since the last visit. Assess risk by recall of exacerbations during the previous year and since the last visit. Recommendations for
adjusting therapy based on level of control are presented in the last row.
Components of Control
Well Controlled Not Well Controlled Very Poorly Controlled
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Impairment
Symptoms ≤2 days/week
≤2 days/week but
not more than
once on each day
≤2 days/week >2 days/week
>2 days/week or
multiple times on
≤2 days/week
>2 days/week Throughout the day
Nighttime awakenings ≤1x/month ≤2x/month >1x/month ≥2x/month 1–3x/week >1x/week ≥2x/week ≥4x/week
Interference with
normal activity
None Some limitation Extremely limited
SABA use for
symptom control
(not to prevent EIB )
≤2 days/week >2 days/week Several times per day
Lung function
FEV
1
(% predicted)
or peak flow
(% personal best)
FEV
1
/FVC
Not applicable
>80%
>80%
>80%
Not applicable
Not applicable
60–80%
75–80%
60–80%
Not applicable
Not applicable
<60%
<75%
<60%
Not applicable
Validated questionnaires
†
ATAQ
ACQ
ACT
Not applicable Not applicable 0
≤0.75
‡
≥20
Not applicable Not applicable 1–2
≥1.5
16–19
Not applicable Not applicable 3–4
Not applicable
≤15
Risk
Asthma exacerbations
requiring oral systemic
corticosteroids
§
0–1/year 2–3/year ≥2/year >3/year ≥2/year
Consider severity and interval since last asthma exacerbation.
Reduction in lung
growth/Progressive loss
of lung function
Not applicable
Evaluation requires long-term
follow-up care.
Not applicable
Evaluation requires long-term
follow-up care.
Not applicable
Evaluation requires long-term
follow-up care.
Treatment-related
adverse effects
Medication side effects can vary in intensity from none to very troublesome and worrisome.
The level of intensity does not correlate to specific levels of control but should be considered in the overall assessment of risk.
Recommended Action
for Treatment
(See “Stepwise Approach for
Managing Asthma Long Term,”
page 7)
The stepwise approach is meant
to help, not replace, the clinical
decisionmaking needed to meet
individual patient needs.
Maintain current step.
Regular follow-up every 1–6 months.
Consider step down if well controlled for at least
3 months.
Step up 1 step
Step up at least
1 step
Step up 1 step
Consider short course of oral systemic corticosteroids.
Step up 1–2 steps.
Reevaluate in 2 weeks to achieve control.
Reevaluate in 2–6 weeks to achieve control.
For children 0–4 years, if no clear benefit observed in 4–6
weeks, consider adjusting therapy or alternative diagnoses.
Before step up in treatment:
Review adherence to medication, inhaler technique, and environmental control. If alternative treatment was used,
discontinue and use preferred treatment for that step. For side effects, consider alternative treatment options.
Abbreviations: ACQ, Asthma Control Questionnaire
©
; ACT, Asthma Control Test
TM
; ATAQ, Asthma Therapy Assessment Questionnaire
©
; EIB, exercise-induced bronchospasm; FVC, forced vital capacity; FEV
1
, forced expiratory volume in 1 second;
SABA, short-acting beta
2
-agonist.
†
Minimal important difference: 1.0 for the ATAQ; 0.5 for the ACQ; not determined for the ACT.
‡
ACQ values of 0.76–1.4 are indeterminate regarding well-controlled asthma.
§
Data are insufficient to link frequencies of exacerbations with different levels of asthma control. Generally, more frequent and intense exacerbations (e.g., requiring urgent care, hospital or intensive care admission, and/or oral corticosteroids)
indicate poorer asthma control.
6
Asthma Care Quick Reference

STEPWISE APPROACH FOR MANAGING ASTHMA LONG TERM
The stepwise approach tailors the selection of medication to the level of asthma severity (see page 5) or asthma control (see page 6).
The stepwise approach is meant to help, not replace, the clinical decisionmaking needed to meet individual patient needs.
At each step: Patient education, environmental control, and management of comorbidities
0–4 years of age
Intermittent
Asthma
Persistent Asthma: Daily Medication
Consult with asthma specialist if step 3 care or higher is required. Consider consultation at step 2.
Preferred
Treatment
†
SABA as
needed
low-dose ICS medium-dose
ICS
medium-dose
ICS
+
either LABA or
montelukast
high-dose ICS
+
either LABA or
montelukast
high-dose ICS
+
either LABA or
montelukast
+
oral corticosteroids
Alternative
Treatment
†
,
‡
cromolyn or
montelukast
If clear benefit is not observed in 4–6 weeks, and medication technique and adherence are satisfactory,
consider adjusting therapy or alternate diagnoses.
Quick-Relief
Medication
SABA as needed for symptoms; intensity of treatment depends on severity of symptoms.
With viral respiratory symptoms: SABA every 4–6 hours up to 24 hours (longer with physician consult). Consider short
course of oral systemic corticosteroids if asthma exacerbation is severe or patient has history of severe exacerbations.
Caution: Frequent use of SABA may indicate the need to step up treatment.
5–11 years of age
Intermittent
Asthma
Persistent Asthma: Daily Medication
Consult with asthma specialist if step 4 care or higher is required. Consider consultation at step 3.
Preferred
Treatment
†
SABA as needed low-dose ICS low-dose ICS
+
either LABA,
LTRA, or
theophylline
(b)
OR
medium-dose
ICS
medium-dose
ICS
+
LABA
high-dose ICS
+
LABA
high-dose ICS
+
LABA
+
oral corticosteroids
Alternative
Treatment
†
,
‡
cromolyn, LTRA,
or theophylline
§
medium-dose ICS
+
either LTRA or
theophylline
§
high-dose ICS
+
either LTRA or
theophylline
§
high-dose ICS
+
either LTRA or
theophylline
§
+
oral corticosteroids
Consider subcutaneous allergen immunotherapy for
patients who have persistent, allergic asthma.
Quick-Relief
Medication
SABA as needed for symptoms. The intensity of treatment depends on severity of symptoms: up to 3 treatments
every 20 minutes as needed. Short course of oral systemic corticosteroids may be needed.
Caution: Increasing use of SABA or use >2 days/week for symptom relief (not to prevent EIB ) generally indicates
inadequate control and the need to step up treatment.
≥12 years of age
Intermittent
Asthma
Persistent Asthma: Daily Medication
Consult with asthma specialist if step 4 care or higher is required. Consider consultation at step 3.
Preferred
Treatment
†
SABA as needed low-dose ICS low-dose ICS
+
LABA
OR
medium-dose ICS
medium-dose
ICS
+
LABA
high-dose ICS
+
LABA
AND
consider
omalizumab for
patients who
have allergies
††
high-dose ICS
+
LABA
+
oral
corticosteroid
§§
AND
consider
omalizumab for
patients who
have allergies
††
Alternative
Treatment
†
,
‡
cromolyn, LTRA,
or theophylline
§
low-dose ICS
+
either LTRA,
theophylline,
§
or zileuton
‡‡
medium-dose ICS
+
either LTRA,
theophylline,
§
or zileuton
‡‡
Consider subcutaneous allergen immunotherapy
for patients who have persistent, allergic asthma.
Quick-Relief
Medication
SABA as needed for symptoms. The intensity of treatment depends on severity of symptoms: up to 3 treatments
every 20 minutes as needed. Short course of oral systemic corticosteroids may be needed.
Caution: Use of SABA >2 days/week for symptom relief (not to prevent EIB ) generally indicates inadequate control
and the need to step up treatment.
Abbreviations: EIB, exercise-induced bronchospasm; ICS, inhaled corticosteroid; LABA, inhaled long-acting beta
2
-agonist; LTRA, leukotriene receptor antagonist; SABA, inhaled
short-acting beta
2
-agonist.
† Treatment options are listed in alphabetical order, if more than one.
‡
If alternative treatment is used and response is inadequate, discontinue and use preferred treatment before stepping up.
§
Theophylline is a less desirable alternative because of the need to monitor serum concentration levels.
Based on evidence for dust mites, animal dander, and pollen; evidence is weak or lacking for molds and cockroaches. Evidence is strongest for immunotherapy with single allergens.
The role of allergy in asthma is greater in children than in adults.
††
Clinicians who administer immunotherapy or omalizumab should be prepared to treat anaphylaxis that may occur.
‡‡
Zileuton is less desirable because of limited studies as adjunctive therapy and the need to monitor liver function.
§§
Before oral corticosteroids are introduced, a trial of high-dose ICS + LABA + either LTRA, theophylline, or zileuton, may be considered, although this approach has not been studied
in clinical trials.
ASSESS
CONTROL:
STEP UP IF NEEDED (first, check medication adherence, inhaler technique, environmental control, and comorbidities)
STEP DOWN IF POSSIBLE (and asthma is well controlled for at least 3 months)
STEP 1
STEP 6
STEP 5
STEP 4
STEP 3
STEP 2
FOLLOW-UP VISITS: ASSESSING ASTHMA CONTROL AND ADJUSTING THERAPY
Level of control (Columns 2–4) is based on the most severe component of impairment (symptoms and functional limitations) or risk (exacerbations). Assess impairment by patient’s or caregiver’s
recall of events listed in Column 1 during the previous 2–4 weeks and by spirometry and/or peak flow measures. Symptom assessment for longer periods should reflect a global assessment,
such as inquiring whether the patient’s asthma is better or worse since the last visit. Assess risk by recall of exacerbations during the previous year and since the last visit. Recommendations for
adjusting therapy based on level of control are presented in the last row.
Components of Control
Well Controlled Not Well Controlled Very Poorly Controlled
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Ages
0–4 years
Ages
5–11 years
Ages
≥12 years
Impairment
Symptoms ≤2 days/week
≤2 days/week but
not more than
once on each day
≤2 days/week >2 days/week
>2 days/week or
multiple times on
≤2 days/week
>2 days/week Throughout the day
Nighttime awakenings ≤1x/month ≤2x/month >1x/month ≥2x/month 1–3x/week >1x/week ≥2x/week ≥4x/week
Interference with
normal activity
None Some limitation Extremely limited
SABA use for
symptom control
(not to prevent EIB )
≤2 days/week >2 days/week Several times per day
Lung function
FEV
1
(% predicted)
or peak flow
(% personal best)
FEV
1
/FVC
Not applicable
>80%
>80%
>80%
Not applicable
Not applicable
60–80%
75–80%
60–80%
Not applicable
Not applicable
<60%
<75%
<60%
Not applicable
Validated questionnaires
†
ATAQ
ACQ
ACT
Not applicable Not applicable 0
≤0.75
‡
≥20
Not applicable Not applicable 1–2
≥1.5
16–19
Not applicable Not applicable 3–4
Not applicable
≤15
Risk
Asthma exacerbations
requiring oral systemic
corticosteroids
§
0–1/year 2–3/year ≥2/year >3/year ≥2/year
Consider severity and interval since last asthma exacerbation.
Reduction in lung
growth/Progressive loss
of lung function
Not applicable
Evaluation requires long-term
follow-up care.
Not applicable
Evaluation requires long-term
follow-up care.
Not applicable
Evaluation requires long-term
follow-up care.
Treatment-related
adverse effects
Medication side effects can vary in intensity from none to very troublesome and worrisome.
The level of intensity does not correlate to specific levels of control but should be considered in the overall assessment of risk.
Recommended Action
for Treatment
(See “Stepwise Approach for
Managing Asthma Long Term,”
page 7)
The stepwise approach is meant
to help, not replace, the clinical
decisionmaking needed to meet
individual patient needs.
Maintain current step.
Regular follow-up every 1–6 months.
Consider step down if well controlled for at least
3 months.
Step up 1 step
Step up at least
1 step
Step up 1 step
Consider short course of oral systemic corticosteroids.
Step up 1–2 steps.
Reevaluate in 2 weeks to achieve control.
Reevaluate in 2–6 weeks to achieve control.
For children 0–4 years, if no clear benefit observed in 4–6
weeks, consider adjusting therapy or alternative diagnoses.
Before step up in treatment:
Review adherence to medication, inhaler technique, and environmental control. If alternative treatment was used,
discontinue and use preferred treatment for that step. For side effects, consider alternative treatment options.
Abbreviations: ACQ, Asthma Control Questionnaire
©
; ACT, Asthma Control Test
TM
; ATAQ, Asthma Therapy Assessment Questionnaire
©
; EIB, exercise-induced bronchospasm; FVC, forced vital capacity; FEV
1
, forced expiratory volume in 1 second;
SABA, short-acting beta
2
-agonist.
†
Minimal important difference: 1.0 for the ATAQ; 0.5 for the ACQ; not determined for the ACT.
‡
ACQ values of 0.76–1.4 are indeterminate regarding well-controlled asthma.
§
Data are insufficient to link frequencies of exacerbations with different levels of asthma control. Generally, more frequent and intense exacerbations (e.g., requiring urgent care, hospital or intensive care admission, and/or oral corticosteroids)
indicate poorer asthma control.
7
Asthma Care Quick Reference

ESTIMATED COMPARATIVE DAILY DOSAGES: INHALED CORTICOSTEROIDS FOR LONG-TERM ASTHMA CONTROL
0–4 years of age 5–11 years of age ≥12 years of age
Daily Dose
Low Medium High
Low
Medium High
Low
Medium High
MEDICATION
Beclomethasone MDI
†
40 mcg/puff
80 mcg/puff
N/A N/A N/A 80–160 mcg
1–2 puffs
2x/day
1 puff 2x/day
>160–320 mcg
3–4 puffs
2x/day
2 puffs 2x/day
>320 mcg
≥3 puffs 2x/day
80–240 mcg
1–3 puffs
2x/day
1 puff am,
2 puffs pm
>240–480 mcg
4–6 puffs
2x/day
2–3 puffs
2x/day
>480 mcg
≥4 puffs
2x/day
Budesonide DPI
†
90 mcg/inhalation
180 mcg/
inhalation
N/A N/A N/A
180–360 mcg
1–2 inhs
†
2x/day
>360–720 mcg
3–4 inhs
†
2x/day
2 inhs
†
2x/day
>720 mcg
≥3 inhs
†
2x/day
180–540 mcg
1–3 inhs
†
2x/day
1 inh
†
am,
2 inhs
†
pm
>540–1,080 mcg
2–3 inhs
†
2x/day
>1,080 mcg
≥4 inhs
†
2x/day
Budesonide Nebules
0.25 mg
0.5 mg
1.0 mg
0.25–0.5 mg
1–2 nebs
†
/day
1 neb
†
/day
>0.5–1.0 mg
2 nebs
†
/day
1 neb
†
/day
>1.0 mg
3 nebs
†
/day
2 nebs
†
/day
0.5 mg
1 neb
†
2x/day
1 neb
†
/day
1.0 mg
1 neb
†
2x/day
1 neb
†
/day
2.0 mg
1 neb
†
2x/day
N/A N/A N/A
Ciclesonide MDI
†
80 mcg/puff
160 mcg/puff
N/A N/A N/A 80–160 mcg
1–2 puffs/day
1 puff/day
>160–320 mcg
1 puff am,
2 puffs pm–
2 puffs 2x/day
1 puff 2x/day
>320 mcg
≥3 puffs 2x/day
≥2 puffs 2x/day
160–320 mcg
1–2 puffs 2x/day
>320–640 mcg
3–4 puffs 2x/day
2 puffs 2x/day
>640 mcg
≥3 puffs 2x/day
Flunisolide MDI
†
80 mcg/puff
N/A N/A N/A 160 mcg
1 puff 2x/day
320–480 mcg
2–3 puffs 2x/day
≥480 mcg
≥4 puffs 2x/day
320 mcg
2 puffs 2x/day
>320–640 mcg
3–4 puffs 2x/day
>640 mcg
≥5 puffs 2x/day
It is preferable to use a higher mcg/puff or mcg/inhalation formulation to achieve as low a number of puffs or inhalations as possible.
†
Abbreviations: DPI, dry powder inhaler (requires deep, fast inhalation); inh, inhalation; MDI, metered dose inhaler (releases a puff of medication); neb, nebule.
8
Asthma Care Quick Reference

0–4 years of age 5–11 years of age ≥12 years of age
Daily Dose Low
Medium High
Low
Medium High
Low
Medium High
MEDICATION
Fluticasone MDI
†
44 mcg/puff
110 mcg/puff
220 mcg/puff
Fluticasone DPI
†
50 mcg/inhalation
100 mcg/inhalation
250 mcg/inhalation
176 mcg
2 puffs 2x/day
N/A
>176–352 mcg
3–4 puffs
2x/day
1 puff 2x/day
N/A
>352 mcg
≥2 puffs
2x/day
N/A
88–176 mcg
1–2 puffs
2x/day
100–200 mcg
1–2 inhs
†
2x/day
1 inh
†
2x/day
>176–352 mcg
3–4 puffs
2x/day
1 puff 2x/day
>200–400 mcg
3–4 inhs
†
2x/day
2 inhs
†
2x/day
>352 mcg
≥2 puffs 2x/day
>400 mcg
>2 inhs
†
2x/day
1 inh
†
2x/day
88–264 mcg
1–3 puffs
2x/day
100–300 mcg
1–3 inhs
†
2x/day
>264–440 mcg
2 puffs 2x/day
1 puffs 2x/day
>300–500 mcg
2 inhs
†
2x/day
1 inh
†
2x/day
>440 mcg
3 puffs 2x/day
≥2 puffs 2x/day
>500 mcg
≥3 inhs
†
2x/day
≥2 inhs
†
2x/day
Mometasone DPI
†
110 mcg/inhalation
220 mcg/inhalation
N/A N/A N/A 110 mcg
1 inh
†
/day
220–440 mcg
1–2 inhs
†
2x/day
1–2 inhs
†
/day
>440 mcg
≥3 inhs
†
2x/day
≥3 inhs
†
divided
in 2 doses
110–220 mcg
1–2 inhs
†
pm
1 inh
†
pm
>220–440 mcg
3–4 inhs
†
pm or
2 inhs
†
2x/day
1 inh
†
2x/day or
2 inhs
†
pm
>440 mcg
≥3 inhs
†
2x/day
≥3 inhs
†
divided
in 2 doses
It is preferable to use a higher mcg/puff or mcg/inhalation formulation to achieve as low a number of puffs or inhalations as possible.
†
Abbreviations: DPI, dry powder inhaler (requires deep, fast inhalation); inh, inhalation; MDI, metered dose inhaler (releases a puff of medication); neb, nebule.
Therapeutic Issues Pertaining to Inhaled Corticosteroids (ICSs) for Long-Term Asthma Control
The most important determinant of appropriate dosing is the clinician’s judgment
of the patient’s response to therapy. The clinician must monitor the patient’s
response on several clinical parameters (e.g., symptoms; activity level; measures of
lung function) and adjust the dose accordingly. Once asthma control is achieved
and sustained at least 3 months, the dose should be carefully titrated down to the
minimum dose necessary to maintain control.
Some doses may be outside package labeling, especially in the high-dose range.
Budesonide nebulizer suspension is the only inhaled corticosteroid (ICS) with
FDA-approved labeling for children <4 years of age.
Metered-dose inhaler (MDI) dosages are expressed as the actuator dose (amount
leaving the actuator and delivered to the patient), which is the labeling required in the
United States. This is different from the dosage expressed as the valve dose (amount
of drug leaving the valve, not all of which is available to the patient), which is used in
many European countries and in some scientific literature. Dry powder inhaler (DPI)
doses are expressed as the amount of drug in the inhaler following activation.
For children <4 years of age: The safety and efficacy of ICSs in children <1 year of
age has not been established. Children <4 years of age generally require delivery of
ICS (budesonide and fluticasone MDI) through a face mask that fits snugly over nose
and mouth to avoid nebulizing in the eyes. Face should be washed after treatment
to prevent local corticosteroid side effects. For budesonide, the dose may be given
1–3 times daily. Budesonide suspension is compatible with albuterol, ipratropium,
and levalbuterol nebulizer solutions in the same nebulizer. Use only jet nebulizers, as
ultrasonic nebulizers are ineffective for suspensions. For fluticasone MDI, the dose
should be divided 2 times daily; the low dose for children <4 years of age is higher
than for children 5–11 years of age because of lower dose delivered with face mask
and data on efficacy in young children.
ESTIMATED COMPARATIVE DAILY DOSAGES:
INHALED CORTICOSTEROIDS FOR LONG-TERM ASTHMA CONTROL (continued)
9
Asthma Care Quick Reference

USUAL DOSAGES FOR OTHER LONG-TERM CONTROL MEDICATIONS*
Medication 0–4 years of age 5–11 years of age ≥12 years of age
Combined Medication (inhaled corticosteroid + long-acting beta
2
-agonist)
Fluticasone/Salmeterol —
DPI
†
100 mcg/50 mcg, 250 mcg/50 mcg, or
500 mcg/50 mcg
MDI
†
45 mcg/21 mcg, 115 mcg/21 mcg, or
230 mcg/21 mcg
Budesonide/Formoterol —
MDI
†
80 mcg/4.5 mcg or 160 mcg/4.5 mcg
Mometasone/Formoterol —
MDI
†
100 mcg/5 mcg
N/A
†
N/A
†
N/A
†
1 inhalation 2x/day; dose
depends on level of
severity or control
2 puffs 2x/day; dose
depends on level of
severity or control
N/A
†
1 inhalation 2x/day; dose
depends on level of severity
or control
2 puffs 2x/day; dose depends
on level of severity or control
2 inhalations 2x/day; dose
depends on severity of asthma
Leukotriene Modifiers
Leukotriene Receptor Antagonists (LTRAs)
Montelukast — 4 mg or 5 mg chewable tablet,
4 mg granule packets, 10 mg tablet
Zafirlukast — 10 mg or 20 mg tablet
Take at least 1 hour before or 2 hours after a meal.
Monitor liver function.
5-Lipoxygenase Inhibitor
Zileuton — 600 mg tablet
Monitor liver function.
4 mg every night at
bedtime (1–5 years of age)
N/A
†
N/A
†
5 mg every night at
bedtime (6–14 years of age)
10 mg 2x/day
(7–11 years of age)
N/A
†
10 mg every night at
bedtime
40 mg daily
(20 mg tablet 2x/day)
2,400 mg daily
(give 1 tablet 4x/day)
Immunomodulators
Omalizumab (Anti IgE
†
) —
Subcutaneous injection, 150 mg/1.2 mL following
reconstitution with 1.4 mL sterile water for injection
Monitor patients after injections; be prepared to treat
anaphylaxis that may occur.
N/A
†
N/A
†
150–375 mg subcutaneous
every 2–4 weeks, depending
on body weight and
pretreatment serum IgE level
Cromolyn
Cromolyn — Nebulizer: 20 mg/ampule
1 ampule 4x/day, N/A
†
<2 years of age
1 ampule 4x/day 1 ampule 4x/day
Methylxanthines
Theophylline —
Liquids, sustained-release tablets, and capsules
Monitor serum concentration levels.
Starting dose 10 mg/kg/
day; usual maximum:
<1 year of age: 0.2 (age in
weeks) + 5 = mg/kg/day
≥1 year of age:
16 mg/kg/day
Starting dose 10 mg/
kg/day; usual maximum:
16 mg/kg/day
Starting dose 10 mg/kg/day
up to 300 mg maximum;
usual maximum:
800 mg/day
Inhaled Long-Acting Beta
2
-Agonists (LABAs) – used in conjunction with ICS
†
for long-term control; LABA is NOT to be used as monotherapy
Salmeterol — DPI
†
50 mcg/blister
Formoterol —DPI
†
12 mcg/single-use capsule
N/A
†
N/A
†
1 blister every 12 hours
1 capsule every 12 hours
1 blister every 12 hours
1 capsule every 12 hours
Oral Systemic Corticosteroids
Methylprednisolone — 2, 4, 8, 16, 32 mg tablets
Prednisolone — 5 mg tablets; 5 mg/5 cc, 15 mg/5 cc
Prednisone — 1, 2.5, 5, 10, 20, 50 mg tablets;
5 mg/cc, 5 mg/5 cc
0.25–2 mg/kg daily
in single dose in a.m.
or every other day as
needed for control
Short course “burst”:
1–2 mg/kg/day, max 60
mg/d for 3–10 days
0.25–2 mg/kg daily
in single dose in a.m.
or every other day as
needed for control
Short course “burst”:
1–2 mg/kg/day, max 60
mg/d for 3–10 days
7.5–60 mg daily in single
dose in a.m. or every other
day as needed for control
Short course “burst”: to
achieve control, 40–60 mg/
day as single or 2 divided
doses for 3–10 days
* Dosages are provided for those products that have been approved by the U.S. Food and Drug Administration or have sufficient clinical trial safety and efficacy data in the
appropriate age ranges to support their use.
† Abbreviations: DPI, dry powder inhaler; IgE, immunoglobulin E; MDI, metered-dose inhaler; N/A, not available (not approved, no data available, or safety and efficacy not
established for this age group).
The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. The clinician
must monitor the patient’s response on several clinical parameters (e.g., symptoms; activity level; measures of lung function) and adjust
the dose accordingly. Once asthma control is achieved and sustained at least 3 months, the dose should be carefully titrated down to the
minimum dose necessary to maintain control.
10
Asthma Care Quick Reference

RESPONDING TO PATIENT QUESTIONS ABOUT INHALED CORTICOSTEROIDS
Questions and varying beliefs about inhaled
corticosteroids (ICSs) are common and may affect
adherence to treatment. Following are some key
points to share with patients and families.
ICSs are the most effective medications for
long-term control of persistent asthma. Because
ICSs are inhaled, they go right to the lungs to
reduce chronic airway inflammation. In general,
ICSs should be taken every day to prevent asthma
symptoms and attacks.
The potential risks of ICSs are well balanced by their
benefits. To reduce the risk of side effects, patients
should work with their doctor to use the lowest dose
that maintains asthma control, and be sure to take the
medication correctly.
• Mouth irritation and thrush (yeast infection),
which may be associated with ICSs at higher
doses, can be avoided by rinsing the mouth and
spitting after ICS use and, if appropriate for the
inhaler device, by using a valved holding chamber
or spacer.
• ICS use may slow a child’s growth rate slightly.
This effect on linear growth is not predictable and
is generally small (about 1 cm), appears to occur
in the first several months of treatment, and is
not progressive. The clinical significance of this
potential effect has yet to be determined. Growth
rates are highly variable in children, and poorly
controlled asthma can slow a child’s growth.
ICSs are generally safe for pregnant women.
Controlling asthma is important for pregnant women
to be sure the fetus receives enough oxygen.
ICSs are not addictive.
ICSs are not the same as anabolic steroids that some
athletes use illegally to increase sports performance.
RESPONDING TO PATIENT QUESTIONS ABOUT LONG-ACTING BETA
2
-AGONISTS
Keep the following key points in mind when
educating patients and families about long-acting
beta
2
-agonists (LABAs).
The addition of LABA (salmeterol or formoterol) to the
treatment of patients who require more than low-dose
inhaled corticosteroid (ICS) alone to control asthma
improves lung function, decreases symptoms, and
reduces exacerbations and use of short-acting
beta
2
-agonists (SABA) for quick relief in most patients
to a greater extent than doubling the dose of ICS.
A large clinical trial found that slightly more deaths
occurred in patients taking salmeterol in a single
inhaler every day in addition to usual asthma therapy*
(13 out of about 13,000) compared with patients taking
a placebo in addition to usual asthma therapy
(3 out of about 13,000). Trials for formoterol in a
single inhaler every day in addition to usual therapy*
found more severe asthma exacerbations in patients
taking formoterol, especially at higher doses, compared
with those taking a placebo added to usual therapy.
Therefore, the Food and Drug Administration placed
a Black Box warning on all drugs containing a LABA.
The established benefits of LABAs added to ICS for the
great majority of patients who require more than low-
dose ICS alone to control asthma should be weighed
against the risk of severe exacerbations, although
uncommon, associated with daily use of LABAs.
LABAs should not be used as monotherapy for
long-term control. Even though symptoms may
improve significantly, it is important to keep taking
ICS while taking LABA.
Daily use should generally not exceed 100 mcg
salmeterol or 24 mcg formoterol.
It is not currently recommended that LABAs be used
to treat acute symptoms or exacerbations.
*
Usual therapy included a wide range of regimens, from those in which no other daily therapy was taken to those in which varying doses of other daily medications were taken.
11
Asthma Care Quick Reference

EDUCATIONAL RESOURCES
National Heart, Lung, and Blood Institute
Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3)
www.nhlbi.nih.gov/guidelines/asthma
Physician Asthma Care Education (PACE): www.nhlbi.nih.gov/health/prof/lung/asthma/pace/
National Asthma Control Initiative (NACI): http://naci.nhlbi.nih.gov
Allergy & Asthma Network Mothers of Asthmatics
800–878–4403
www.aanma.org
American Academy of Allergy, Asthma,
and Immunology
414–272–6071
www.aaaai.org
American Academy of Pediatrics
847–434–4000
www.aap.org
American Association of Respiratory Care
972–243–2272
www.aarc.org
American College of Chest Physicians
847–498–1400
www.chestnet.org
American College of Allergy, Asthma & Immunology
847–427–1200
www.acaai.org
American Lung Association
800–LUNG–USA (800–586–4872)
www.lungusa.org
American School Health Association
800–445–2742
www.ashaweb.org
Asthma and Allergy Foundation of America
800–7–ASTHMA (800–727–8462)
http://aafa.org
Centers for Disease Control and Prevention
800–CDC–INFO (800–232–4636)
www.cdc.gov/asthma
Environmental Protection Agency/
Asthma Community Network
www.asthmacommunitynetwork.org
800–490–9198 (to order EPA publications)
www.epa.gov/asthma/publications.html
National Association of School Nurses
240–821–1130
www.nasn.org
For more information contact:
NHLBI Information Center
P.O. Box 30105
Bethesda, MD 20824–0105
Phone: 301–592–8573
Fax: 301–592–8563
Web site: www.nhlbi.nih.gov
NIH Publication No. 12-5075
Originally Printed June 2002
Revised September 2012
