
Genetic technologies
1
—the ability to manipulate and trans-
form the properties of cells, seeds, microbes, insects, plants,
animals and even humans—are pushing the frontiers of
science and oers us new hope for disease control and cure.
is eld has come a long way since Gregor Mendel, the
father of genetics, rst postulated the rule of heredity in the
1850s. Genetic technologies are changing the way we produce
food, improving crop yield and preventing catastrophic losses
from droughts, oods and pests. ey also are oering new
solutions for ghting cancer and many hereditary diseases,
improving quality of life and life expectancy. In addition,
genetic technologies are increasingly used in criminal justice
systems to exonerate the innocent and convict the guilty. Such
technologies, moreover, have given rise to genetic genealogy,
allowing people to nd their ethnic roots.
While the upsides of genetic technologies are promising,
we also need to consider their downside risks. Access to gene
therapies to combat diseases, for example, may be limited to
those who can aord them, potentially increasing inequality
in health outcomes within and across countries. Genomic
research that serves to identify pre-existing conditions can
potentially deprive patients from health insurance and medical
care. Genetic technologies may exacerbate productivity gaps
in agriculture, disadvantaging small farmers, especially in
developing countries, who cannot access or aord genet-
ically modied seeds. Moreover, there can be unintended
health consequences of genetically modied crop produc-
tion, including increased risks of contamination and loss
of biodiversity.
e downside risks can be even uglier. Genetic
modications can potentially lead to the production of
“designer babies” and super-humans and fundamentally alter
the human species. Genomic research can be weaponized
to target and harm specic population groups. e legal,
ethical and moral boundaries of using genetic technologies
are increasingly unclear, creating opportunities for their
misuse and abuse. Weighing potential benets against risks
thus remains an urgent challenge. is Frontier Technology
Quarterly discusses the potential of genetic technologies for
improving health and agricultural productivity, two important
goals of the 2030 Agenda for Sustainable Development, the
1 e term broadly encompasses both genetics—the study of genes and
their role passing traits or conditions from one generation to another, and
genomics—the study of all of a person’s genes (the genome), including
interactions of those genes with each other and with the person’s
environment. Genomics includes the scientic study of complex diseases
such as heart disease, asthma, diabetes, and cancer because these diseases
are typically caused more by a combination of genetic and environmental
factors than by individual genes. Genomics is oering new possibilities
for therapies and treatments for some complex diseases, as well as new
diagnostic methods (source: www.genome.gov).
risks posed by these technologies for increasing inequities in
health outcomes and their potential misuse and abuse.
I. The good
Genetic technologies are oering new solutions for disease
control, prevention and cure. ey are now being used to
diagnose and treat complex diseases such as heart disease,
asthma, diabetes and cancer. Genetic technologies may also
soon allow us to eradicate malaria, a major health menace in
many developing countries.
Eradicating malaria
Malaria is one of the most severe public health epidemics
in sub-Saharan Africa and large swaths of Asia and Latin
America (Figure 1). It is a leading cause of death, especially
in Africa, where a quarter of the population remains at risk
of contracting the disease. According to the World Health
Organization (WHO), one child dies from malaria every
two minutes. In 2017, there were an estimated 219 million
malaria cases worldwide and 435,000 deaths.
2
e social
2 https://www.who.int/news-room/fact-sheets/detail/malaria
World Economic and Social Survey 2018: Frontier Technology for
Sustainable Developmenta agship publication of the United
Nations Department of Economic and Social Aairs (DESA)
generated considerable interest in new technologies and
their development impacts. Inspired by this strong interest,
the Economic Analysis and Policy Division has undertaken to
produce quarterly reviews on frontier technologies, delving
deeper into specic aspects of a new technology. The series
will identify challenges and raise many questionsand answer
a fewwhile motivating policy research in DESA and
beyond. This second edition of the series discusses genetic
technologies. The quarterly reviews will be shared and discussed
in development policy seminars and social media platforms to
enrich policy discourse on frontier technologies.
Economic Analysis and Policy Division w Department of Economic and Social Affairs
FRONTIER TECHNOLOGY QUARTERLY
Playing with genes: The good, the bad and the ugly
May
2019
Kristinn Helgason, Marcelo LaFleur, and Hamid Rashid, Economic
Analysis and Policy Division (EAPD) of UN DESA, authored this
issue of Frontier Technology Quarterly. Nazrul Islam, Alex Julca,
Hiroshi Kawamura, and Mariangela Parra-Lancourt provided
useful comments on the draft. Research support was provided by
Nicole Hunt and Linying He. Hamid Rashid, in his capacity as the
Chief of Development Research Branch in EAPD, supervised the
production. The views and opinions expressed herein are those
of the authors and do not necessarily reect those of the United
Nations Secretariat.

2
FRONTIER TECHNOLOGY QUARTERLY
and economic costs of malaria are signicant. Governments and
societies bear the cost burden of health facilities, personnel,
drugs, public health campaigns and interventions to ght and
contain malaria, diverting scarce resources away from productive
economic activities.
Gene drives
3
to combat malaria promise large improvement
in health outcomes in many developing countries, particularly for
young children and pregnant women who are most vulnerable to
the disease. ey can alter the life cycle of the parasite or eradicate
it completely. Computer models—simulating the gene drive and
other interventions—estimate that malaria could be eliminated
from large regions within two decades. e speed and eectiveness
of gene drives also make the technique potentially dangerous, as it
may trigger unforeseen mutations or aect other insect species.
Developing resilient food crops
Food production is often susceptible to adverse weather, ecological
and soil conditions. Genetically engineered (GE) or genetically
modied organisms (GMO)
4
are allowing the production of
more resilient crop varieties. A new cost eective and easy-to-use
technique, known by its acronym CRISPR, has revolutionized the
process of decoding and precisely editing genetic information of
3 A gene drive is a genetic engineering technology—adding, deleting, disrupting,
or modifying genes—to rapidly spread a particular genetic trait to an entire
ospring population. A gene drive can alter or eliminate an entire species.
4 A genetically modied organism (GMO) is an organism in which one or more
genes (called transgenes) have been introduced into its genetic material from
another organism using recombinant DNA technology. For example, the
genes may be from a dierent kingdom (such as from a bacterium to a plant)
or a dierent species within the same kingdom (e.g. from one plant species to
another).
organisms.
5
e International Rice Research Institute (IRRI), for
example, has genetically engineered the Stress-Tolerant Rice for
Africa and South Asia (STRASA), which maintains normal yield
even when submerged in ood water. By 2017, more than 8 million
farmers in South Asia were using the STRASA rice variety.
Adoption of genetically modied crops has been rapid, espe-
cially in the United States (Figure 2) where GMO crops account for
more than 80% of planted acres. Brazil, China and India are also
leading producers of GMO crops. While GMO crops have made
food production more resilient to pesticide, infestation, drought or
ooding, they have also raised concerns about direct and indirect
costs of production, including cost of seeds, land degradation, envi-
ronmental sustainability and safety.
Large farms in developed economies can usually aord the
scale and intensity of GMO crop production, potentially disad-
vantaging small farmers in developing countries. Not surprisingly,
GMO crop production remains concentrated in a handful of coun-
tries, with the United States accounting for 40% of the planted
crop land (Figure 3).
6
is raises additional concerns regarding
competition, global supply chain, crop prices, and food security for
millions in many developing countries. Large-scale GMO crops are
increasingly disadvantaging small farmers in developing countries,
who are unable to compete in the market place on either price or
quantity. e GMO seed production is also concentrated among
few large rms who enjoy enormous market power to control
price and supply of seeds, making small farmers vulnerable to
market manipulation.
5 http://www.fao.org/3/MX160en/mx160en.pdf
6 Author’s compilation from http://www.isaaa.org/resources/publications/
briefs/53/download/isaaa-brief-53-2017.pdf
Figure 1
Countries with indiginous cases of malaria in 2000 and their status by 2017
Source: World Health Organization

3
FRONTIER TECHNOLOGY QUARTERLY
Advancing human genome research
Technological breakthroughs are lowering the cost of gene
sequencing and editing, but gene therapies are still too expensive
for most people. e cost of sequencing genes has declined dramat-
ically—from nearly $9 million in 2007 to just $1,100 per genome
in 2017—due to a revolutionary technology called Next Generation
Sequencing.
7
is drastic reduction in cost, though still prohibi-
tively expensive for average income-earners in many developing
countries, has made sequencing and studying genes feasible for
many countries. It has encouraged competition among countries
to establish themselves as leaders in genomics, pursuing a range
of objectives (Table 1).
8
While countries are prioritizing genomic
research, international cooperation is also playing a critical role.
e Human Heredity and Health in Africa (H3Africa) initiative,
an example of successful collaboration in genetic research, directs
funding from the National Institutes of Health (NIH) and the
Wellcome Trust to research sites across Africa that study genomics,
environmental determinants of common illnesses, disease suscepti-
bility and drug responses in African populations.
II. The bad
e high price tag of many genetic technologies means that not
everyone will benet. e cost of gene therapies for rare diseases as
approved in the United States and Europe can range from $373,000
to $1 million per patient per year. While genomics is shaping
the future of medicine, the research is often targeted for certain
population groups in mind, especially wealthy people who possess
the ability to pay.
7 https://www.genome.gov/sequencingcostsdata/
8 Authors compilation from https://www.clinicalomics.com/topics/biomarkers-
topic/biobanking/10-countries-in-100k-genome-club/
A widening genomics-divide in healthcare
According to the WHO, only 10 per cent of the US$70 billion
health research spending worldwide is focused on the health needs
of 90 per cent of the world’s population. Large pharmaceutical
companies primarily focus their eorts on protable markets and as
a result, only 13 of the 1,223 new drugs introduced between 1975
and 1996 targeted tropical diseases.
is reects not only the entrenched divide in research and
development (R&D) expenditures between developed and devel-
oping countries but also dierential priorities in medical research.
Pharmaceutical rms in developed countries dominate
genomic innovations, raising concerns of a “genomics divide”
that can further exacerbate existing inequality in health outcomes
between rich and poor nations. e Food and Drug Administration
in the United States, for example, has received over 100 applications
for new gene therapies in 2017. e 721 on-going gene therapy trials
will treat 1,000 rare diseases, which means only a small number
of patients will benet from such gene therapies, keeping the
price of treatment out of reach for most people. It will
nevertheless remain important to establish clear guidelines for
genetic research and access to genomic information, to ensure that
the beneciaries of various genomic research represent the diversity
of the entire population.
e market demand for nding cures for rare diseases explain
the rapid proliferation of gene therapies in the United States and
other developed economies. ere are, however, positive spillover
eects of the high cost of—and the high pay-o from—gene ther-
apies. As researchers look for cure for one rare disease, they will
invariably expand our understanding of gene level behavior and
Figure 2
Adoption of genetically engineered crops in the United
States, 1996-2018
Source: US Department of Agriculture, https://www.ers.usda.gov/data-products/
adoption-of-genetically-engineered-crops-in-the-us.aspx
Figure 3
Global Share (%) of GMO-planted Croplands in 2017
Source: UN DESA
40%
12%
7%
6%
9%
Global Share (%) of GMO-planted Croplands in 2017
United States
Brazil
Argentina
Canada
India
ROW
26%
4
FRONTIER TECHNOLOGY QUARTERLY
potentially lead us to cures for other intractable diseases. While
each life matters, societies will need to make tough choices, consid-
ering the opportunity costs of spending millions, if not billions
of private and public money, to treat rare diseases that aect very
small population groups. Making a few better o, while ignoring
the medical needs of millions, will only exacerbate inequities in
health outcomes, even in the most developed countries. e rich
living longer and healthier, while the poor lack basic healthcare,
will further entrench alienation and societal discontent.
III. The ugly
Gene editing has opened a Pandora’s Box. While it presents great
hopes for curing disease and eliminating hunger, gene editing is still
imprecise, which could lead to inadvertent and undesirable changes
to a genome. ere are also concerns regarding the unknown, long-
term safety of gene editing.
Ethical concerns
ere are growing concerns about how to govern the use of germline
editing technologies in the health sector. Germline editing refers to
genetic modications that can be inherited by an ospring. is
process raises many ethical questions, especially if gene editing
is used to address a genetic diagnosis of an unborn child, where
any o-target edits can evolve quickly. e discussion around the
usefulness and the risks of germline editing came to the spotlight
after a Chinese scientist announced he had edited the genetic
material of two babies prior to their birth. e changes were meant
to be benign, making the unborn babies less susceptible to HIV
infection. ere are, however, concerns that the genetic sequence
targeted in this procedure may also aect brain development.
It also raises questions about what constitutes “informed consent”.
How can a future person have a voice on genetic changes that
will aect them throughout their lives, and perhaps passed on
to their ospring? Regulation of gene editing research involving
human embryos has gained added urgency in recent months as
news broke out that the rst genetically edited children have
been born in China. Some 30 countries already have in place
legislation that directly or indirectly bars the use of germline
editing technologies.
Introducing genetic changes in a population, even if
successful, can also lead to unforeseen ecological impact. Delivering
a genetic mutation for combating malaria—using a gene drive into
the wild—is risky and the harm caused by a disease such as malaria
must be balanced against the possible ecological side-eects of
the proposed solution. Once released, the mutation will spread as
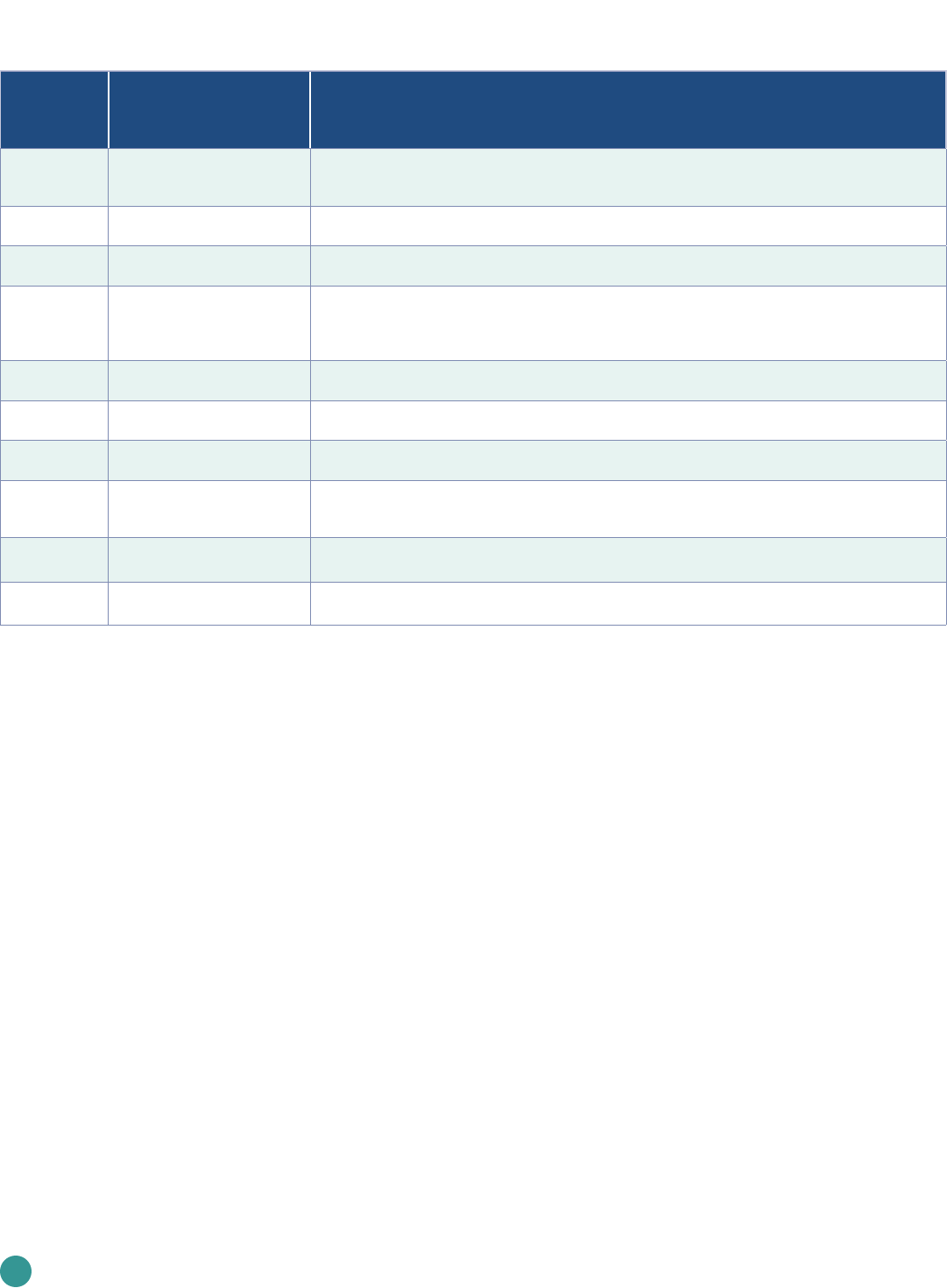
Table 1
Countries establishing themselves as leaders in genomics: select projects and their objectives
Country Initiative Objective
Australia
Australian Genomics Health
Futures Mission
Develop national standards and protocols to enhance data gathering and analysis; promote the
value of genomics to the broader community; and encourage government partnerships with
philanthropists and businesses
China 100,000 Genome Project
Study how Chinese population transform from health to disease, environmental impacts, and the
interactions between environmental factors and genes, and its influence on people’s health
Estonia
Personalized Medicine
Programme
Develop genotypes that will enable personalized reports for use in everyday medical practice
through the national e-health portal
France France Génomique 2025
Integrate genomic medicine into routine patient care and establish a genomic medicine industry
to fuel economic growth. By 2020, France aims to have increased its annual sequencing capacity to
235,000 genomes, of which 175,000 are to come from cancer patients, and the remaining 60,000
from rare disease patients
Japan
Initiative on Rare and
Undiagnosed Diseases
Develop innovative drug candidates by targeting novel, single pathological mutations, apply new
NGS-based genome analyses to cases that remain unsolved, and facilitate international data sharing
Saudi Arabia
Saudi Human Genome
Program
Study more than 5,000 inherited diseases using more than 10,000 samples from Saudi patients with
inherited diseases that resulted in identification of more than 2,000 variants underlying the diseases
Turkey Turkish Genome Project
Sequence the genomes of 100,000 Turkish nationals and increase that number to 1 million genomes
by 2023
United Arab
Emirates
United Arab Emirates—
Dubai Genomics
Sequence all of its 3 million residents. Dubai Genomics is one of numerous projects within the Dubai
Future Foundation’s “Dubai 10X Initiative,” launched to catapult the UAE 10 years ahead of the rest
of the world
United
Kingdom
100,000 Genome Project
Incorporate genome sequencing in routine healthcare through the Genomic Medicine Service (GMS).
Sequenced 71,095 whole genomes
United States All of Us Research Program Glean health and wellness data from 1 million or more Americans

5
FRONTIER TECHNOLOGY QUARTERLY
designed and may not be recalled or easily disabled. ere is also
the possibility that eliminating a species may unleash unforeseen
consequences. e genetic mutation itself may somehow aect a
benign insect species, such as bees, causing untold harm to the
ecosystem that supports farming and other plant life.
ere are also social and national sovereignty considerations
as mutations will not be conned to certain geographical regions or
national borders. As genetic technologies continue to advance and
as the technical barriers to solving many challenges fall, social and
sovereignty concerns remain and are accentuated. Genetic drives
to combat malaria, for example, will likely need a regional, and
possibly a global, agreement among countries.
IV. Finding appropriate balance
Countries will need to nd appropriate balance between incentiv-
izing advances in genetic technologies and managing their intended
benets and unintended consequences. e balance will rest on
three pillars: (1) consent and privacy; (2) information sharing and
intellectual property rights; and, (3) ethical boundaries. First and
foremost, genetic research involving humans must require informed
consent. e privacy and safety of a research subject or beneciary
must be protected to facilitate further progress in genetic research.
e immortal Hela cells of Henrietta Lacks, an African American
cancer patient who died in 1951, have been a major source of
genetic research worldwide for more than 60 years. Since the
1950s, scientists have grown as much as 50 million metric tons of
her cells, and there are almost 11,000 patents involving HeLa cells.
Yet neither Henrietta nor her family members ever consented to the
research, raising concerns for her privacy. ere was a public outcry
when a group of scientists published the HeLa genome in 2013
without the consent of her descendants. By the time the researchers
removed the genome from public view it had been downloaded at
least 15 times.
e privacy concerns of individuals must be balanced
against the need for sharing genetic data broadly to facilitate
research collaboration. Achieving this balance is particularly
dicult in genomics given that DNA sequence is unique to
each person, making it impossible to fully anonymize the data.
ere is, however, broad consensus in the research community
that DNA sequencing data should be made public within 24
hours of being generated, as agreed in the Bermuda Declaration.
e open access policy largely explains the rapid advances in human
genome research during the past 15 years.
e privacy concern and open access policy, however, can
come into conict with intellectual property protection typically
aorded to innovation. Patents encourage innovation and incentiv-
izes investments in research and yet it can also stie further inno-
vation, limiting access to critical genetic information stored behind
patent protection. e future of genomic research will also hinge on
intellectual property rights information and sharing of information.
e earliest genetic patents were issued in 1982, which opened the
debate whether DNA sequencing was a mere discovery or met the
denition of invention. In 2013, the US Supreme Court concluded
that DNA in its natural form cannot be patented. However, gene
therapies and other genetic interventions typically enjoy patent
protection, explaining the high price tag. International cooperation
in genomic research will need to address patent protection issues to
make gene therapies more accessible and aordable.
Ethical concerns will remain the most critical challenge
for managing the risks in genetic research. Genetic research is
becoming more commonplace and yet most genetic tests are not
regulated, even in the United States. e claims of many genetic
results are not independently veried, making them susceptible to
fraud and manipulation. More importantly, there is no interna-
tionally agreed guidelines for human genome research. Informed
consent, privacy protection and patent rights can still be insu-
cient to prevent unethical genetic research. e genetic research
community generally adheres to the Declaration of Helsinki
that the World Medical Association adopted in 1964 to guide
medical research with human subjects. e code of ethics embodied
in the Declaration protects individual subjects from potential
harm but does not necessarily spell out the responsibility of the
researcher to take into account the risks on third parties and other
spillover eects.
Select country approaches to regulate genetic technologies
Canada, strongly inuenced by public outcry over the production by British scientists of a cloned sheep called “Dolly”, decided to ban
and criminalize human cloning research in 2004. In Germany, the creation, use, and harvesting of embryonic cells for basic research
are also prohibited. In France, the modication of the human genome may be undertaken for preventive, diagnostic or therapeutic
purposes only. The United Kingdom, on the other hand, allowed in 2016 the application of genetic technology in research on human
embryos. In the Republic of Korea, laws prohibit genetic experimentation with and modication of human embryos, including any
product that alters genes. The concerns about “o-target eects” of genetic technologies, meaning that not all copies of the target
gene are edited, have also further complicated the regulatory process in a number of countries.
Because of deep-rooted concerns that developments in the eld of genetic technologies may outpace ethical guidelines, there
is strong public support across countries in all regions for subjecting regulation in this area to extensive stakeholder consultations.
In Germany, for example, the National Academy of Sciences convened a gene editing debate in 2017 that included members of the
public as well as ocials from various federal ministries. In Australia, the Oce of the Gene Technology Regulator, in 2017, invited
the public to provide comments during a review of the country’s gene technology regulation. In Qatar has adopted a consultative
approach to policymaking on issues relating to bioethics of new genetic technologies. The Qatar consultations have involved scien-
tists, industry experts, government representatives and scholars in Islamic jurisprudence.

6
FRONTIER TECHNOLOGY QUARTERLY
International cooperation
A number of prominent scientists have called on governments to
adopt more specic standards and principles at the intergovern-
mental level to guide the regulation of genetic technologies. is
includes a group of eighteen scientists and ethicists from seven
countries, who in a 13 March 2019 article in the Nature magazine
called on governments to declare a 5-year global moratorium on
all clinical uses of germline editing until the technical, scientic,
medical, societal, ethical and moral implications have been more
thoroughly discussed and understood.
Earlier this year, the Director-General of WHO established a
new advisory committee on developing global standards for govern-
ance and oversight of human genome editing. e committee agreed
that it would be irresponsible at this time for anyone to proceed
with the clinical application of human germline editing. It has also
requested WHO to immediately begin working on a central registry
on human genome editing research. Over the next two years, the
committee will conduct a series of meetings and consultations
with all relevant stakeholders with a view to providing recommen-
dations for a governance framework that is scalable, sustainable
and appropriate for use at the international, regional, national
and local levels.
An eective global governance framework is an imperative
for ensuring safe and sound application of genetic technologies and
making them accessible to all. e stakes are high when it comes
to the unsafe and unethical application of genetic technologies as
discussed in this FTQ. e World Health Organization and the
Food and Agriculture Organization of the United Nations will
need to continue playing a pivotal role in promoting greater under-
standing of the risks and benets of genetic technologies and devel-
oping internationally agreed norms and standards for their safe and
ethical use. e quest for reaching a global consensus on ethical use
of genetic technologies should not encourage the Member States to
look for the least common denominator solutions and de minimis
standards. e standards should be suciently aspirational and
forward-looking—guided by the principles of the UN Charter
and the Universal Declaration of Human Rights—given that they
will aect not only this current generation but also our future
generations. A misstep will be too costly for humanity.
References
Carlson, RV, KM Boyd and DJ Webb (2004). The revi-
sion of the Declaration of Helsinki: past, present and
future. Br J Clin Pharmacol, vol. 57, No. 6, pp. 695–713.
doi:10.1111/j.1365-2125.2004.02103.x
Center for Disease Control and Prevention (2019). Malaria
fact sheet.
CGIAR (2017). CGIAR Research Program on Rice Agri-Food
System (RICE) 2017 Annual Report.
Food and Agriculture Organization of the United Nations (2012).
Statistical Yearbook 2012.
Food and Agriculture Organization of the United Nations (2018).
Commission on Genetic Resources for Food and Agriculture,
Report of Meeting, 25-27 July.
Global Knowledge Center on Crop Biotechnology (2015). Pocket
K No. 18, February.
Helmy, Mohamed, Mohamed Awad, and Kareem A. Mosa 2016.
Limited resources of genome sequencing in developing
countries: challenges and solutions, Applied & Translational
Genomics 9 (Supplement C):15–19. https://doi.org/10.1016/j.
atg.2016.03.003.
ISAAA (2017). Biotech Crop Adoption Surges as Economic Benefits
Accumulate in 22 Years. Brief No. 53-2017. Ithaca, NY.
National Human Genome Research Institute (2017). DNA
sequencing costs. https://www.genome.gov
Trouiller, Patrice, and Piero L. Olliaro (1998). “Drug Development
Output from 1975 to 1996: What Proportion for Tropical
Diseases?” International Journal of Infectious Diseases: IJID;
Hamilton 3 (2): 61.
United Nations (2018). World Economic and Social Survey 2018:
Frontier technologies for sustainable development. Sales No. E.18.
II.C.1, chap. 3.
Wade, Nicolas (2018). Giving malaria a deadline, The New York
Times, 24 September.
World Health Organization. 2019. Global Health Observatory
Data Repository 2019. http://apps.who.int/gho/data/
node.home.
