
2024
ALZHEIMER’S DISEASE
FACTS AND FIGURES
SPECIAL REPORT
MAPPING A BETTER
FUTURE FOR DEMENTIA
CARE NAVIGATION

Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
About this report
2024 Alzheimer’s Disease Facts and Figures is a statistical
resource for U.S. data related to Alzheimer’s disease, the
most common cause of dementia. Background and context
for interpretation of the data are contained in the Overview.
Additional sections address prevalence, mortality and
morbidity, caregiving, the dementia care workforce, and
the use and costs of health care and services. The Special
Report provides a comprehensive look into dementia care
navigation, revealing significant insights into the experiences
and challenges faced by caregivers and health care
workers in helping people living with Alzheimer’s or other
dementia navigate the health care system.
The statistics, facts, figures, interpretations and statements made in this report
are based on currently available data and information as cited in this report, all of
which are subject to revision as new data and information become available.

12024 Alzheimer’s Disease Facts and Figures
Specific information in this year’s
Alzheimer’s Disease Facts and Figures includes:
Brain changes that occur with Alzheimer’s disease (page 8).
Risk factors for Alzheimer’s dementia (page 15).
Number of Americans with Alzheimer’s dementia nationally (page 22) and for each state (page 26).
Lifetime risk for developing Alzheimer’s dementia (page 28).
Proportion of women and men with Alzheimer’s and other dementias (page 28).
Number of deaths due to Alzheimer’s disease nationally (page 35) and for each state (page 38),
and death rates by age (page 40).
Number of family caregivers, hours of care provided, and economic value of unpaid care nationally
(page 43) and for each state (page 47).
The impact of caregiving on caregivers (page 48).
The impact of COVID-19 on dementia caregiving (page 55).
Members of the paid workforce involved in diagnosing, treating and caring for people with
Alzheimer’s or other dementias (page 59).
Expected home health and personal care aide job growth, 2020-2030 (page 63).
National cost of care for individuals with Alzheimer’s or other dementias, including costs paid by
Medicare and Medicaid and costs paid out of pocket (page 71).
Medicare payments for people with dementia compared with people without dementia (page 72).
Care navigator services that would be valuable to dementia caregivers (page 105).
The Appendices detail sources and methods used to derive statistics in this report.
When possible, specific information about Alzheimer’s disease is provided; in other cases, the reference may be
a more general one of “Alzheimer’s or other dementias.” This report keeps the racial and ethnic terms and other
population identifiers used in source documents when describing study findings.

2 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Contents
Prevalence
Prevalence of Alzheimer’s
and Other Dementias in
the United States 22
Underdiagnosis of
Alzheimer
’
s and Other
Dementias in the
Primary Care Setting 23
Prevalence of Subjective
Cognitive Decline 23
Prevalence Estimates
24
Estimates of the
Number of People with
Alzheimer’s Dementia by
State and County
28
Incidence of
Alzheimer’s Dementia
28
Lifetime Risk of
Alzheimer’s Dementia
28
Differences Between Women
and Men in the Prevalence
and Risk of Alzheimer’s and
Other Dementias
28
Racial and Ethnic Differences
in the Prevalence and Risk
of Alzheimer’s and Other
Dementias
29
Risk for Alzheimer’s
and Other Dementias in
Sexual and Gender
Minority Groups
31
Trends in the Prevalence
and Incidence of Alzheimer’s
Dementia Over Time
32
Looking to the Future
33
Overview
Alzheimer’s Disease
or Dementia? 5
Brain Changes of
Alzheimer’s Disease 8
Alzheimer’s Disease
Continuum 10
Mixed Dementia 12
When Dementia-Like
Symptoms Are Not
Dementia 12
Treatments 12
Actions to Proactively
Manage Dementia 14
Risk Factors for
Alzheimer’s Dementia 15
Looking to the Future 20
Caregiving
Unpaid Caregivers 43
Caregiving and Women 44
Race, Ethnicity and
Dementia Caregiving 45
Caregiving Tasks 45
Duration of Caregiving 46
Hours of Unpaid Care
and Economic Value of
Caregiving 48
Health and Economic
Impacts of Alzheimer’s
Caregiving 48
Interventions Designed
to Assist Caregivers 53
COVID-19 and Dementia
Caregiving 55
Trends in Dementia
Caregiving 56
A National Strategy
to Support Family
Caregivers 57
Mortality and Morbidity
Deaths from
Alzheimer’s Disease 35
The Effect of the COVID-19
Pandemic on Deaths from
Alzheimer’s Disease 36
Public Health Impact of
Deaths from Alzheimer’s
Disease 39
State-by-State Deaths
from Alzheimer’s 39
Alzheimer’s Death Rates 39
Duration of Illness from
Diagnosis to Death and Time
Spent in Nursing Home 40
The Burden of
Alzheimer’s Disease 40
Looking to the Future 41

3Contents
Use and Costs of
Health Care, Long-Term
Care and Hospice
Total Cost of Health Care
and Long-Term Care 71
Use and Costs of
Health Care Services 73
Use and Costs of
Long-Term Care Services 76
Medicare Does Not
Cover Long-Term Care
in a Nursing Home 81
Use and Costs of
Health Care and
Long-Term Care Services
by Race and Ethnicity 87
The COVID-19 Pandemic
and Health Care
Utilization and Costs 88
Use of Potentially Avoidable
Health Care Services 89
Looking to the Future 90
Caregiver Burden and
Stress Are Compounded
by the Complexity of
Dementia Care 94
Nationwide Movement
to Improve Care While
Reducing Strain on
Caregivers 94
What is GUIDE? 94
Navigators as Dementia
Care Wayfinders 95
Prioritizing Person-
Centered Care in Dementia
Care Navigation 96
Awareness and
Understanding of
Dementia Care Navigation:
Caregiver and Health Care
Workforce Surveys 96
Key Findings 96
Survey Design and
Research Methods 98
Dementia Care
Survey Results 99
Cultural Competency is
Fundamental for Dementia
Care Navigation 102
Non-Physician
Health Care Workforce
Survey Results 104
A Path Forward:
Revolutionizing Dementia
Care With Person-
Centered Navigation 111
Conclusion 114
Workforce
Screening and
Diagnosing Workforce 59
Medical Treatment and
Care Team Workforce 62
Collaborative Workforce
Models for Dementia
Care Management 64
Direct Care Workforce 64
Impact of COVID-19
on the Workforce 66
Dementia-Friendly
Initiatives and the
Community-Based
Workforce 67
Looking to the Future 67
Appendices
End Notes 115
References 118
Special Report – Mapping
a Better Future for
Dementia Care Navigation

Overview
Alzheimer’s begins 20 years
or more before memory loss
and other symptoms develop.

5Overview
Alzheimer’s disease is a type of brain disease, just as
coronary artery disease is a type of heart disease. It is
caused by damage to nerve cells (neurons) in the brain.
The brain’s neurons are essential to all human activity,
including thinking, talking and walking.
Individuals with mild symptoms often may continue to
work, drive and participate in their favorite activities,
with occasional help from family members and friends.
However, Alzheimer’s disease is a progressive disease,
meaning it gets worse with time. How quickly it
progresses and what abilities are affected vary from
person to person. As time passes, more neurons are
damaged and more areas of the brain are affected.
Increased help from family members, friends and
professional caregivers is needed to carry out everyday
activities. Eventually, people may need help with activities
of daily living. These are activities a person typically
performs without assistance, including getting into and
out of a bed or chair, bathing, dressing, using the toilet,
eating and grooming.
Individuals living with Alzheimer’s dementia may develop
changes in mood, personality or behavior. One behavior
of special concern is wandering. For the person with
dementia, wandering is likely an intentional effort to
reach a destination. However, they may not be able to
retrace their steps and may become lost. Wandering puts
individuals at risk of significant injury and death.
9
Alzheimer’s Disease or Dementia?
Many people wonder what the difference is
between Alzheimer’s disease and dementia.
Dementia is an overall term for a particular
group of symptoms. The characteristic
symptoms of dementia are difficulties with
memory, language, problem-solving and
other thinking skills that affect a person’s
ability to perform everyday activities.
Changes to the brain cause dementia, and
many different brain changes can lead to
dementia (see Table 1, page 6).
Alzheimer’s disease is one cause of dementia.
The brain changes of Alzheimer’s disease
include the excessive accumulation of the
protein fragment beta-amyloid and an
abnormal form of the protein tau, as well as
damage to and destruction of neurons. The
brain changes of Alzheimer’s disease are
the most common contributor to dementia.
Dementia caused by Alzheimer’s disease is
called Alzheimer’s dementia.
In Alzheimer’s disease, the neurons
damaged first are those in parts of the
brain responsible for memory, language
and thinking, which is why the first symptoms
tend to be memory, language and thinking
problems. Although these symptoms are
new to the individual affected, the brain
changes that cause them are thought to
begin 20 years or more before symptoms
start.
1-8
When symptoms become severe
enough to interfere with a person’s ability
to perform everyday tasks, a person is said
to have Alzheimer’s dementia.
Eventually, the neuronal damage of Alzheimer’s extends
to parts of the brain that enable basic bodily functions
such as walking and swallowing. Because of mobility
limitations, individuals may spend most of their time in a
wheelchair or on a bed. This loss of mobility, along with
cognitive limitations, means they often require around-
the-clock care. Ultimately, Alzheimer’s disease is fatal,
although many people die of other conditions before
Alzheimer’s becomes fatal. Studies indicate that people
age 65 and older survive an average of four to eight years
after a diagnosis of Alzheimer’s dementia, yet some live as
long as 20 years.
10-18
Many factors influence how long
6 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Alzheimer’s disease
Accumulation of the protein beta-amyloid outside neurons and twisted strands of the
protein tau inside neurons are hallmarks. They are accompanied by the death of neurons
and damage to brain tissue. Inflammation and atrophy of brain tissue are other changes.
Cerebrovascular disease
Blood vessels in the brain are damaged and/or brain tissue is injured from not
receiving enough blood, oxygen or nutrients. People with these changes who
develop dementia symptoms are said to have vascular dementia.
Frontotemporal
degeneration (FTD)
Nerve cells in the front and temporal (side) lobes of the brain die and the lobes shrink.
Upper layers of the cortex soften. Abnormal amounts or forms of tau or transactive
response DNA-binding protein (TDP-43) are present.
Cause Brain changes
*This table describes the most common causes of dementia. Emerging causes such as limbic-predominant age-related TDP-43 encephalopathy
(LATE) are under active investigation.
Hippocampal sclerosis (HS)
HS is the shrinkage and hardening of tissue in the hippocampus of the brain.
The hippocampus plays a key role in forming memories. HS brain changes are often
accompanied by accumulation of the misfolded protein TDP-43.
Lewy body disease
Lewy bodies are abnormal aggregations (or clumps) of the protein alpha-synuclein
in neurons. When they develop in a part of the brain called the cortex, dementia
can result. This is called dementia with Lewy bodies or DLB.
Mixed pathologies
When an individual shows the brain changes of more than one cause of dementia,
“mixed pathologies” are considered the cause. When these pathologies result in
dementia symptoms during life, the person is said to have mixed dementia or
mixed etiology dementia.
Parkinson’s disease (PD)
Clumps of the protein alpha-synuclein appear in an area deep in the brain called the
substantia nigra. These clumps are thought to cause degeneration of the nerve cells
that produce the chemical dopamine.
29
As PD progresses, alpha-synuclein can also
accumulate in the cortex.
Common Causes of Dementia*
Table
1

7Overview
Alzheimer’s is the most common cause of dementia,
accounting for an estimated 60% to 80% of cases. Most
individuals also have the brain changes of one or more other
causes of dementia.
21,22
This is called mixed pathologies,
and if recognized during life is called mixed dementia.
Difficulty remembering recent conversations, names or events;
apathy; and depression are often early symptoms. Communication
problems, confusion, poor judgment and behavioral changes may
occur next. Difficulty walking, speaking and swallowing are common
in the late stages of the disease.
About 5% to 10% of individuals with dementia show evidence
of vascular dementia alone.
21,22
However, it is more common
as a mixed pathology, with most people living with dementia
showing the brain changes of cerebrovascular disease and
Alzheimer’s disease.
21,22
Slowed thoughts or impaired ability to make decisions, plan
or organize may be the initial symptoms, but memory may also
be affected. People with vascular dementia may become less
emotional and have difficulty with motor function, especially
slow gait and poor balance.
About 60% of people with FTD are ages 45 to 60.
23
In a
systematic review, FTD accounted for about 3% of dementia
cases in studies that included people 65 and older and about
10% of dementia cases in studies restricted to those younger
than 65.
24
Typical early symptoms include marked changes in personality
and behavior and/or difficulty with producing or comprehending
language. Unlike Alzheimer’s, memory is typically spared in the
early stages of disease.
HS is present in about 3% to 13% of people with dementia.
25
It often occurs with the brain changes of other causes of
dementia. An estimated 0.4% to 2% of dementia cases are
due to HS alone.
25
The most pronounced symptom of HS is memory loss, and
individuals are often misdiagnosed as having Alzheimer’s disease.
HS is a common cause of dementia in individuals age 85 or older.
About 5% of older individuals with dementia show evidence
of DLB alone, but most people with DLB also have the brain
changes of Alzheimer’s disease.
26
Early symptoms include sleep disturbances, well-formed visual
hallucinations and visuospatial impairment. These symptoms
may change dramatically throughout the day or from day to day.
Problems with motor function (similar to Parkinson’s disease) are
common. Memory loss may occur at some point in the disease.
More than 50% of people diagnosed with Alzheimer’s
dementia who were studied at Alzheimer’s Disease Research
Centers had mixed dementia.
22
In community-based studies,
the percentage is considerably higher.
21
Mixed dementia is
most common in people age 85 or older.
27,28
Symptoms vary depending on the combination of brain
changes present.
A systematic review found that 3.6% of dementia cases were due
to PD and 24.5% of people with PD developed dementia.
30
Problems with movement (slowness, rigidity, tremor and changes
in gait) are common symptoms of PD. Cognitive symptoms may
develop later in the disease, typically years after movement symptoms.
Percentage of dementia cases Symptoms
8 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
individuals live after receiving a diagnosis. They include
age at diagnosis, how far the disease has progressed at
diagnosis, whether the individual has other health
conditions such as diabetes or kidney disease that may
limit remaining lifespan and complicate care and
treatment, and whether the individual has mixed dementia
— the brain changes of not only Alzheimer’s disease but
also another type of dementia.
There is no proven way to prevent Alzheimer’s disease,
and there is currently no cure. However, because of the
large number of people living with Alzheimer’s and other
dementias worldwide (more than 55 million)
19
and the
devastating effect of dementia on individuals, families,
communities and health care systems, finding ways to
prevent, slow, better manage and cure Alzheimer’s and
other dementias is a top priority for research centers
around the globe.
Brain Changes of Alzheimer’s Disease
A healthy adult brain has billions of neurons, each
with long, branching extensions. These extensions
enable individual neurons to form connections with
other neurons. At such connections, called synapses,
information flows in tiny bursts of chemicals that are
released by one neuron and taken up by another
neuron. The brain contains trillions of synapses.
They allow signals to travel rapidly through the brain.
These signals are the basis of memories, thoughts,
sensations, emotions, movements and skills.
Over the years, researchers have identified many
changes in the brain that may interfere with chemical
signaling and lead to problems with thinking, learning
and everyday function that arise as a result of
Alzheimer’s disease. The accumulation of the protein
fragment beta-amyloid into clumps (called beta-amyloid
plaques) outside neurons and the accumulation of an
abnormal form of the protein tau (called tau tangles)
inside neurons are two of several brain changes
associated with Alzheimer’s disease.
Beta-amyloid and tau have different roles in Alzheimer’s.
Plaques and smaller accumulations of beta-amyloid may
damage neurons by interfering with neuron-to-neuron
communication at synapses. Inside neurons, tau tangles
block the transportation of nutrients and other
molecules essential for the normal function and survival
of neurons while harming connections between neurons.
Beta-amyloid and tau accumulation are followed by
damage to and destruction of neurons (called
neurodegeneration) and other brain cells.
Neurodegeneration, along with beta-amyloid and tau
accumulation, are key features of Alzheimer’s disease.
The presence of toxic beta-amyloid and tau proteins is
believed to activate immune system cells in the brain
called microglia. Microglia try to clear the toxic proteins
and debris from dead and dying cells. Chronic inflammation
may set in when the microglia can't keep up with all that
needs to be cleared.
Another brain change associated with Alzheimer’s disease
is atrophy (decreased brain volume) resulting from
neurodegeneration and other factors. While some degree
of brain atrophy is common in older age, even in people
who are cognitively healthy, atrophy is accelerated in
people with Alzheimer’s dementia.
20
Normal brain function
is further compromised by decreases in the brain's ability to
metabolize glucose, its main fuel.
Timing of Brain Changes
Researchers have gained insight into the timing of
these brain changes. Among people with rare genetic
mutations that cause Alzheimer’s disease for whom
long-term data have been collected, researchers have
found that levels of beta-amyloid significantly increased
starting 22 years before symptoms were expected to
develop (individuals with these genetic mutations usually
develop symptoms at the same or nearly the same age
as their parent with Alzheimer’s).
5
In another study,
abnormal levels of the neurofilament light chain protein,
a biomarker of neurodegeneration, were also found to
start 22 years before symptoms were expected to
develop.
7
A third group of researchers found that levels
of different types of tau protein increase when beta-
amyloid clumps together as amyloid plaques, and that
levels of these types of tau increase as early as two
decades before the characteristic mature tau tangles of
Alzheimer’s disease appear.
8
Researchers also found that
glucose metabolism starts decreasing 18 years before
expected symptom onset, and brain atrophy begins
13 years before expected symptom onset.
5
Brain Changes as Biomarkers
These brain changes are biomarkers of Alzheimer’s
disease. Biomarkers are biological changes that can be
measured to indicate the presence or absence of a
disease or the risk of developing a disease. For example,
the level of glucose in blood is a biomarker of diabetes,
and cholesterol level is a biomarker of cardiovascular
disease risk. Great progress has been made in measuring
Alzheimer’s disease biomarkers. For example, we can
now identify abnormal levels of beta-amyloid and tau in
cerebrospinal fluid (CSF, the fluid surrounding the brain),
and an imaging technique known as positron emission
tomography (PET) can produce pictures showing where
beta-amyloid and tau have accumulated in the brain. In
addition, many research groups are working on blood
tests for Alzheimer’s disease. If these blood tests prove
effective they could simplify and greatly speed-up
diagnosis of Alzheimer’s.

9Overview
Signs of Alzheimer’s Dementia Typical Age-Related Changes
Memory loss that disrupts daily life: One of the most common signs of Alzheimer’s dementia,
especially in the early stage, is forgetting recently learned information. Others include asking
the same questions over and over, and increasingly needing to rely on memory aids (for example,
reminder notes or electronic devices) or family members for things that used to be handled on
one’s own.
Sometimes forgetting names or
appointments, but remembering
them later.
Challenges in planning or solving problems: Some people experience changes in their ability to
develop and follow a plan or work with numbers. They may have trouble following a familiar recipe
or keeping track of monthly bills. They may have difficulty concentrating and take much longer to
do things than they did before.
Making occasional errors
when managing finances or
household bills.
Difficulty completing familiar tasks: People with Alzheimer’s often find it hard to complete daily
tasks. Sometimes, people have trouble driving to a familiar location, organizing a grocery list or
remembering the rules of a favorite game.
Occasionally needing help to
use microwave settings or record
a television show.
Confusion with time or place: People living with Alzheimer’s can lose track of dates, seasons
and the passage of time. They may have trouble understanding something if it is not happening
immediately. Sometimes they forget where they are or how they got there.
Getting confused about the
day of the week but figuring it
out later.
Trouble understanding visual images and spatial relationships: For some people, having vision
problems is a sign of Alzheimer’s. They may also have problems judging distance and determining
color and contrast, causing issues with driving.
Vision changes related to
cataracts.
New problems with words in speaking or writing: People living with Alzheimer’s may have trouble
following or joining a conversation. They may stop in the middle of a conversation and have no idea
how to continue or they may repeat themselves. They may struggle with vocabulary, have trouble
naming a familiar object or use the wrong name (e.g., calling a watch a “hand clock”).
Sometimes having trouble
finding the right word.
Misplacing things and losing the ability to retrace steps: People living with Alzheimer’s may put
things in unusual places. They may lose things and be unable to go back over their steps to find
them. They may accuse others of stealing, especially as the disease progresses.
Misplacing things from time
to time and retracing steps to
find them.
Decreased or poor judgment: Individuals may experience changes in judgment or decision-making.
For example, they may use poor judgment when dealing with money or pay less attention to
grooming or keeping themselves clean.
Making a bad decision or
mistake once in a while, such
as neglecting to schedule an oil
change for a car.
Withdrawal from work or social activities: People living with Alzheimer’s disease may experience
changes in the ability to hold or follow a conversation. As a result, they may withdraw from hobbies,
social activities or other engagements. They may have trouble keeping up with a favorite sports
team or activity.
Sometimes feeling uninterested
in family and social obligations.
Changes in mood, personality and behavior: The mood and personalities of people living with
Alzheimer’s can change. They can become confused, suspicious, depressed, fearful or anxious.
They may be easily upset at home, at work, with friends or when out of their comfort zones.
Developing very specific ways
of doing things and becoming
irritable when a routine is disrupted.
*For more information about the symptoms of Alzheimer’s, visit alz.org.
Signs of Alzheimer’s Dementia Compared With Typical Age-Related Changes*
Table
2
Table
2

Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).10
Preclinical Alzheimer’s Disease
In this phase, individuals may have measurable brain changes that indicate the earliest signs of Alzheimer’s disease,
but they have not yet developed symptoms such as memory loss or difficulty thinking. Examples of Alzheimer's brain
changes that might be detected in this phase include abnormally increased levels and distribution of beta-amyloid and
tau and decreased metabolism of glucose as shown on positron emission tomography (PET) scans, as well as changes
in tau protein in cerebrospinal fluid (CSF).
45-47
When the early changes of Alzheimer’s disease occur, the brain
compensates for them, enabling individuals to continue to function normally.
Although research settings have the tools and expertise to identify some of the early brain changes of Alzheimer’s
disease, additional research is needed to fine-tune the tools’ accuracy before they become available for widespread use
in hospitals, doctors’ offices and other clinical settings. It is important to note that not all individuals with evidence of
Alzheimer’s-related brain changes go on to develop symptoms of MCI or dementia due to Alzheimer’s disease before
their deaths, even if they live for many years or decades after these biomarkers are detected.
48-50
On this continuum, there are three broad phases:
preclinical Alzheimer’s disease, mild cognitive impairment
(MCI) due to Alzheimer’s disease and dementia due to
Alzheimer’s disease, also called Alzheimer’s dementia
(see Figure 1).
40-43
The Alzheimer’s dementia phase is
further broken down into mild, moderate and
severe dementia.
While we know the Alzheimer’s disease continuum
starts with preclinical Alzheimer’s disease (no
symptoms) and ends with severe Alzheimer’s dementia
(severe symptoms), how long individuals spend in each
part of the continuum varies. The length of each part
of the continuum is influenced by age, genetics and
other factors.
44
The progression of Alzheimer’s disease from
brain changes that are unnoticeable by the
person affected to brain changes that cause
problems with memory and thinking, and
eventually physical disability, is called the
Alzheimer’s disease continuum.
Alzheimer’s Disease Continuum
Symptoms interfere
with most everyday
activities
Symptoms interfere
with many everyday
activities
Symptoms interfere
with some everyday
activities
Very mild symptoms
that may not interfere
with everyday activities
No symptoms but
possible biological
changes in the brain
Preclinical AD
Mild Cognitive
Impairment Due to AD
Mild Moderate
Dementia Due to ADDementia Due to AD
*Although these arrows are of equal size, the components of the AD continuum are not equal in duration.
Severe
Dementia Due to AD
Alzheimer’s Disease (AD) Continuum*
Figure
1

11Overview
MCI Due to Alzheimer’s Disease
People with MCI due to Alzheimer’s disease have biomarker evidence of Alzheimer’s brain changes plus new
but subtle symptoms such as problems with memory, language and thinking. These cognitive problems may
be noticeable to the individual, family members and friends, but not to others, and they may not interfere with
the individual’s ability to carry out everyday activities.
Everyone who develops Alzheimer’s dementia first experiences MCI, although it might not be recognized or
diagnosed because of the subtlety of symptoms. Among those with MCI, about 15% develop dementia after two
years.
51
About one-third develop dementia due to Alzheimer’s within five years.
52
However, some individuals
with MCI do not have additional cognitive decline or revert to normal cognition. Among population-based studies,
a systematic review and meta-analysis reported a reversion rate of 26%.
53
Identifying which individuals with MCI
are more likely to develop dementia is a major goal of current research.
Dementia Due to Alzheimer’s Disease
Dementia due to Alzheimer’s disease is characterized by noticeable memory, language, thinking or behavioral
symptoms that impair a person’s ability to function in daily life, combined with biomarker evidence of Alzheimer’s-
related brain changes. As Alzheimer’s disease progresses, individuals commonly experience multiple types of
symptoms that change with time. These symptoms reflect the degree of damage to neurons in different parts
of the brain. The pace at which symptoms of dementia advance from mild to moderate to severe differs from
person to person.
Mild Alzheimer’s Dementia
In the mild stage of Alzheimer’s dementia, most individuals are able to function independently in many areas but are
likely to require assistance with some activities to maximize independence and remain safe. They may still be able to
drive, work and participate in their favorite activities. They may need more time to complete common daily tasks.
Paying bills and making financial decisions may be especially challenging. The U.S. Social Security Administration
notes that people living with dementia are at an especially high risk of becoming victims of fraud and financial
abuse.
54
This may be because handling finances is a particularly complex cognitive activity made even harder by
declines in executive function. Executive function comprises the higher-level cognitive skills used to control and
coordinate other cognitive abilities and behaviors.
55
Declines in executive function can play out as difficulty
planning, organizing and carrying out tasks, as well as poor judgment, socially inappropriate behavior, and inability to
understand how one’s behavior or choices affect others.
56
Impaired executive function not only makes it challenging
for individuals with Alzheimer’s dementia to manage finances, but may also make them especially vulnerable to
financial abuse and scams because their ability to discern between well-intentioned and ill-intentioned behavior and
language in others may be diminished.
Moderate Alzheimer’s Dementia
In the moderate stage of Alzheimer’s dementia, which is often the longest stage, individuals experience more
problems with memory and language, are more likely to become confused, and find it harder to complete multistep
tasks such as bathing and dressing. They may become incontinent at times, begin to have problems recognizing
loved ones, and start showing personality and behavioral changes, including suspiciousness and agitation.
Severe Alzheimer’s Dementia
In the severe stage of Alzheimer’s dementia, individuals’ ability to communicate verbally is greatly diminished, and
they are likely to require around-the-clock care. Because of damage to areas of the brain involved in movement,
individuals may be unable to walk. As a result, they may spend most of their time in a wheelchair or bed. This loss of
mobility increases their vulnerability to physical complications including blood clots, skin infections and sepsis (a
condition that triggers body-wide inflammation that can result in organ failure). Damage to areas of the brain that
control swallowing makes it difficult to eat and drink. This can result in individuals swallowing food into the trachea
(windpipe) instead of the esophagus (food pipe). As a result, food particles may be deposited in the lungs and cause a
type of lung infection called aspiration pneumonia. Aspiration pneumonia is a contributing cause of death among
many individuals with Alzheimer’s dementia (see Mortality and Morbidity section, page 34).

12 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Mixed Dementia
Many people with dementia have brain changes
associated with more than one cause.
21, 31-36
This is called
mixed dementia. Some studies report that the majority
of people with the brain changes of Alzheimer’s disease
also have the brain changes of a second cause of
dementia on autopsy.
21, 22
One autopsy study showed
that of 447 older people who were believed to have
Alzheimer’s dementia when they died, only 3% had the
brain changes of Alzheimer’s disease alone, while 15%
had the brain changes of an entirely different cause of
dementia, and 82% had the brain changes of Alzheimer’s
disease plus at least one other cause of dementia.
21
Studies suggest that mixed dementia is the norm,
not just for those diagnosed with Alzheimer’s dementia but
also for those diagnosed with other types of dementia.
37, 38
Mixed dementia is especially common at advanced
ages.
31, 39
For example, those age 85 or older are more
likely than those younger than 85 to have evidence of
two or more causes of dementia.
27, 28
Having Alzheimer’s
brain changes plus brain changes of another type of
dementia increases one’s chances of having dementia
symptoms in one’s lifetime compared with having
Alzheimer’s brain changes alone.
21, 31
Mixed dementia
may also account for the wide variety of memory and
thinking problems experienced by people living with
dementia. It is currently not possible to determine with
certainty which symptoms are due to which dementia.
Treatments
Drug Treatments
A total of eight drugs are available for the treatment
of Alzheimer’s disease. Two of these drugs change the
underlying biology of Alzheimer’s and slow cognitive and
functional decline in some individuals. A third such drug
was under review by the FDA for potential approval at
press time. Six additional drugs have been approved that
treat the symptoms of Alzheimer’s dementia.
Treatments to Slow Alzheimer’s Disease
The drugs aducanumab and lecanemab change the
underlying biology of Alzheimer’s disease and delay
disease progression. They do this by helping remove
plaques and a form of beta-amyloid called protofibrils
that plays a role in the development of beta-amyloid
plaques. Earlier this year, the manufacturer of
aducanumab announced that the drug was being
discontinued.
57
The manufacturer said aducanumab
is being discontinued in order for the company "to
reprioritize its resources in Alzheimer's disease."
Aducanumab is not being discontinued for reasons
related to safety or efficacy. People who are now
receiving the drug as part of a clinical trial will continue
to have access to aducanumab until May 1, 2024, and
aducanumab will continue to be available until
November 1, 2024, for people who are now receiving
it by prescription.
Focusing on lecanemab, clinical trials of the drug showed
moderate slowing of cognitive and functional decline
in individuals with mild cognitive impairment (MCI) or
mild dementia due to Alzheimer’s.
58
Only individuals with
MCI or mild dementia due to Alzheimer’s and evidence of
beta-amyloid buildup in the brain based on brain imaging
or CSF analysis were eligible to participate in clinical
trials of lecanemab. Lecanemab is not a cure for
Alzheimer’s disease and not appropriate for all individuals
living with Alzheimer’s. Safety and effectiveness have
only been established in individuals living with MCI due
to Alzheimer’s disease and mild dementia due to
Alzheimer’s disease.
It’s important to note that while clinical trials showed
statistically significant differences in cognitive outcomes
between people randomized to receive lecanemab and
those randomized to receive placebo, the benefits of
lecanemab in the short term may be imperceptible to
those receiving them. Because lecanemab has been
approved fairly recently, we don’t know its effectiveness
over the long term, although extension studies, in which
people who volunteer for a clinical trial continue to
receive treatment after a trial is completed, are underway.
When Dementia-Like Symptoms
Are Not Dementia
It is important to note that some individuals
have dementia-like symptoms without the
progressive brain changes of Alzheimer’s or
other degenerative brain diseases. Causes of
dementia-like symptoms include depression,
untreated sleep apnea, delirium, side
effects of medications, Lyme disease, thyroid
problems, head injury, blood clots or tumors
in the brain, certain vitamin deficiencies
and excessive alcohol consumption. Unlike
Alzheimer’s and other dementias, the
dementia caused by these conditions often
may be reversed with treatment.
The differences between normal age-related
cognitive changes and the cognitive
changes of Alzheimer’s disease can be subtle
(see Table 2, page 9). People experiencing
cognitive changes should seek medical
help to determine if the changes are normal
for their age, are reversible, or may be a
symptom of Alzheimer’s or another dementia.
13Overview
Anti-amyloid treatments such as aducanumab and
lecanemab can have side effects. They can cause serious
allergic reactions as well as amyloid-related imaging
abnormalities (ARIA), infusion-related reactions,
headaches and falls.
ARIA is a common side effect that does not usually cause
symptoms but can be serious. It is typically a temporary
swelling in areas of the brain and usually resolves over
time. Some people may also have small spots of bleeding
in or on the surface of the brain along with swelling.
Most people with swelling who experience ARIA do not
have symptoms. Those who do experience symptoms
of ARIA may have headache, dizziness, nausea, confusion
and vision changes. Management of ARIA may include
discontinuation of the medication either temporarily
or indefinitely.
Individuals with two copies of the APOE-e4 gene are
at higher risk of developing ARIA.
59
The FDA encourages
APOE-e4 testing before starting treatment. Prior to
testing, doctors should discuss with patients the risk of
ARIA and the implications of genetic testing results.
These are not all the possible side effects. Individuals
should talk with their doctors to develop a treatment
plan that is right for them, including weighing the
benefits and risks of all approved therapies.
Appropriate use recommendations have been
developed to guide physicians in determining which
individuals should and should not receive treatment with
lecanemab.
59
The recommendations also discuss ARIA
monitoring and management, key points to share with
individuals living with dementia and their care partners,
and incorporating treatments into clinical practice.
A variety of other treatments targeting the underlying
biology of Alzheimer’s disease are being developed.
They address many of the known brain changes
associated with Alzheimer’s disease, including but not
limited to tau accumulation, inflammation, altered cell
metabolism and oxidative stress (damage from toxic
oxygen molecules).
60,61
As of January 1, 2023,
156 clinical trials were underway testing additional
disease-modifying therapies.
62
Treatments to Address Cognitive and Behavioral Symptoms
Five of these eight drugs — donepezil, rivastigmine,
galantamine, memantine and memantine combined with
donepezil — are aimed at treating cognitive symptoms.
They do not affect the underlying brain changes that
cause Alzheimer’s, nor do they slow or stop the course
of the disease. With the exception of memantine, they
treat symptoms by increasing the amount of chemicals
called neurotransmitters in the brain. Neurotransmitters
help brain cells communicate with each other. Memantine
protects the brain from excessive levels of a
neurotransmitter called glutamate, which overstimulates
neurons and can damage them. These five drugs may
have side effects, such as headaches and nausea. These
are not all the possible side effects. As with lecanemab,
individuals should talk with their doctors to develop a
treatment plan that is right for them, including weighing
the benefits and risks of all approved therapies.
One of the eight drugs, brexpiprazole, has been approved
by the FDA to treat agitation that can occur in
Alzheimer’s. Agitation is common in Alzheimer’s disease,
with 60% of people with MCI and 76% of people with
Alzheimer’s dementia experiencing agitation.
63
Brexpiprazole is thought to lessen agitation through its
effects on dopamine and serotonin receptors in the
brain. Brexpiprazole is also FDA-approved for the
treatment of major depressive disorder. It's important to
note that brexpiprazole is an atypical antipsychotic drug,
Atypical antipsychotics have been associated with an
increased risk of stroke and death in older patients with
dementia-related psychosis.
64, 65
Non-drug interventions
should be tried first.
In addition to these eight drugs, the drug suvorexant,
approved for insomnia, has been shown in clinical trials
to be effective in treating problems with falling asleep
and staying asleep that can occur in people with mild to
moderate Alzheimer's disease. Suvorexant inhibits the
activity of orexin, a type of neurotransmitter involved
in the sleep-wake cycle. Possible side effects include,
but are not limited to, impaired alertness and motor
coordination (including impaired driving), worsening of
depression or suicidal thinking, complex sleep behaviors
(such as sleep-walking and sleep-driving), sleep paralysis
and compromised respiratory function.
Why insomnia and other sleeping problems occur in
people living with Alzheimer's is unclear. However,
researchers have found that Alzheimer’s brain changes
disrupt the sleep-wake cycle, leading to increased sleep
fragmentation and wakefulness and decreased slow-
wave sleep.
66
Researchers have also found that sleep
abnormalities accelerate the accumulation of beta-
amyloid and release of toxic tau in the brain, increasing
the risk of dementia. In this way, sleep problems may be
bidirectional, with Alzheimer’s disease increasing the risk
of sleep disturbances and sleep disturbances increasing
the risk of Alzheimer’s.
66, 67
More research is needed to
better understand the relationship between sleep
abnormalities and Alzheimer’s. About one-quarter of
people with dementia have problems sleeping, and the
problems can worsen as the disease progresses.
68
As of January 1, 2023, 31 clinical trials were underway
testing additional agents to treat Alzheimer’s cognitive,
behavioral and neuropsychiatric symptoms.
62

14 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Non-Drug Treatments
There are also non-drug treatments for the symptoms
of Alzheimer’s disease. Non-drug treatments do not
change the underlying biology of the disease. They are
often used with the goals of maintaining or improving
cognitive function, overall quality of life and engagement,
and the ability to perform activities of daily living.
Non-drug treatments include physical activity,
memory and orientation exercises, music- and art-based
therapies, and many others. Non-drug treatments may
be used with a more specific goal of reducing behavioral
and psychological symptoms such as depression, apathy,
wandering, sleep disturbances, agitation and aggression.
For example, a review and analysis of nonpharmacologic
treatments for agitation and aggression in people with
dementia concluded that non-drug interventions
seemed to be more effective than pharmacologic
interventions for reducing aggression and agitation.
69
Risk Factors for Alzheimer’s Dementia
The vast majority of people who develop Alzheimer’s
dementia are age 65 or older. This is called late-onset
Alzheimer’s dementia. Experts believe that Alzheimer’s
dementia, like other common chronic diseases and
conditions, develops as a result of multiple factors
rather than a single cause. Exceptions are cases of
Alzheimer’s related to trisomy 21 in Down syndrome
and rare cases of Alzheimer’s disease related to specific
genetic mutations.
Age, Genetics and Family History
The greatest risk factors for Alzheimer’s dementia are
older age,
70, 71
genetics — especially the e4 form of the
apolipoprotein E (APOE) gene
72, 73
— and having a family
history of Alzheimer’s dementia.
74-77
Age
Age is the greatest of these three risk factors. The
percentage of people with Alzheimer’s dementia
increases dramatically with age. Five percent of people
age 65 to 74, 13.2% of people age 75 to 84, and 33.4%
of people age 85 or older have Alzheimer’s dementia
(see Prevalence section, page 22). The aging of the
population, including the baby-boom generation, will
significantly increase the number of people in the
United States with Alzheimer’s dementia.
78
However,
it is important to note that Alzheimer’s dementia is
not a normal part of aging, and older age alone is not
sufficient to cause Alzheimer’s dementia.
79
Actions for the Person Living with Dementia
and Their Caregivers
These actions include:
• Becoming educated about the disease.
• Maintaining a sense of self and relationships
with others.
- Identifying and participating in activities that
are meaningful and bring purpose to one’s life.
- Identifying opportunities to connect with
others living with dementia and their caregivers
and participating in related activities.
• Planning for the future, including future
health care needs, changes in employment
and financial changes.
Actions for Health Care Providers and Other
Members of the Health Care Workforce
These actions include:
• Appropriate use of available treatment options.
• Effective management of coexisting conditions.
• Coordination of care among health care providers,
other health care professionals and lay caregivers.
• Directing family caregivers to resources to help
them learn how to manage the day-to-day needs
of the individual living with dementia.
To learn more, see the Caregiving section (page 42)
and Workforce section (page 58). Visit alz.org to
learn more about Alzheimer’s disease, as well as
practical information for living with Alzheimer’s and
being a caregiver.
Actions to Proactively Manage Dementia
Proactive management of Alzheimer’s and other dementias can improve the quality of life of affected
individuals and their caregivers. Proactive management includes actions by the person living with dementia
and their caregivers and actions by health care providers and other members of the health care workforce.

15Overview
Genetics
Researchers have found many genes that increase or
decrease the risk of Alzheimer’s dementia. In fact, in
2022 researchers identified 31 new genes that appear
to affect biological processes known to be at play in
Alzheimer’s disease.
80
Of the many genes that increase
risk, APOE-e4 has the strongest impact on risk of
late-onset Alzheimer’s dementia. APOE provides the
blueprint for a protein that transports cholesterol in
the bloodstream. Everyone inherits one of three forms
(alleles) of the APOE gene — e2, e3 or e4 — from
each parent, resulting in six possible APOE pairs: e2/e2,
e2/e3, e2/e4, e3/e3, e3/e4 and e4/e4.
Having the e4 form of APOE increases one’s risk of
developing Alzheimer’s dementia compared with having
the e3 or e2 forms but does not guarantee that an
individual will develop Alzheimer’s dementia. Having the
e2 form may decrease one’s risk compared with having
the e3 or e4 form. Individuals with the e2 form who
develop Alzheimer’s dementia generally do so later in life
than those without the e2 form. The e3 form is thought
to have a neutral effect on Alzheimer’s dementia risk.
In general, the risk of developing Alzheimer’s dementia
increases with inheriting one copy of the e4 form and
increases further still with inheriting two copies of the
e4 form, compared with inheriting only copies of the e2
or e3 forms.
79-81
For example, based on data from a
study in Europe and a study in the United States, of
*Data for APOE pairs in other populations are not available.
Percentages do not total 100 due to rounding.
†
Study provided a percentage for women and a percentage for men.
Percentages represent the range for the two.
Created from data from Rajan et al
93
and Kataoka et al.
94
APOE Pair
African
Americans
European
Americans
American
Indians
†
e3/e3 45.2 63.4 71.6 - 73.2
e3/e4 28.6 21.4 22.7 - 23.9
e3/e2 15.1 10.2 2.6 - 3.0
e2/e4 5.7 2.4 0.5
e4/e4 4.5 2.4 1.0 - 1.2
e2/e2 0.7 0.2 0.0 - 0.1
Percentage of African Americans, European Americans
and American Indians with Specified APOE Pairs*
This report keeps the racial, ethnic and other
population identifiers used in source documents
when describing findings from specific studies.
Table
3
people age 65-69, the risk of developing dementia by
the early to mid-80s was 5% to 7% among those with no
copies of the e4 form, 15% to 16% among those with
one copy, and 31% to 40% among those with two
copies.
82
In addition, those with the e4 form are more
likely to have beta-amyloid accumulation and Alzheimer’s
dementia at a younger age than those with the e2 or e3
forms of the APOE gene.
83
A meta-analysis including 20 published articles
describing the frequency of the e4 form among people
in the United States who had been diagnosed with
Alzheimer’s dementia found that 56% had one copy of
the APOE-e4 gene, and 11% had two copies of the
APOE-e4 gene.
84
Another study found that among
1,770 diagnosed individuals from 26 Alzheimer’s Disease
Research Centers across the United States, 65% had at
least one copy of the APOE-e4 gene.
85
Most of the research to date associating APOE-e4
with increased risk of Alzheimer’s dementia has studied
White individuals. Studies of this association in Black and
Hispanic populations have had inconsistent results. For
example, some have found that having the e4 allele did
not increase risk among Black people,
86-88
while other
studies have found that it significantly increased risk.
89-92
In addition, researchers have found differences in the
frequency of APOE pairs among racial and ethnic
groups. For instance, data show that a higher percentage
of African Americans have at least one copy of the e4
allele (see Table 3) than European American and
American Indian people.
86, 87, 93, 94
Among individuals of
African ancestry who have one copy of e3 and one of e4,
those with a particular variant of e3 called R145C are at
increased risk of developing Alzheimer’s dementia
compared with those who do not have this variant.
95
Researchers have also found that another genetic
factor, the ATP-binding cassette transporter (ABCA7)
protein, doubles the risk of Alzheimer’s dementia in
Black people with ABCA7 compared with Black people
without ABCA7.
90
To better understand inconsistencies in the effect of
APOE-e4 in Hispanic/Latino groups, one research team
analyzed the effect of APOE-e4 in 4,183 individuals from
six Latino backgrounds: Central American, Cuban,
Dominican, Mexican, Puerto Rican and South American.
96

16 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
They found that the effect of APOE-e4 on cognitive
decline differed among groups, suggesting that factors
related to geographic background and genetic ancestry
may alter the extent to which APOE-e4 contributes to
cognitive decline.
These inconsistencies point to the need for more
research to better understand the genetic mechanisms
involved in Alzheimer’s risk among different racial and
ethnic groups.
Trisomy in Down Syndrome
In Down syndrome, an individual is born with three
copies of chromosome 21 (called trisomy 21) instead of
two. People with Down syndrome have an increased risk
of developing Alzheimer’s dementia, and this is believed
to be related to trisomy 21. Chromosome 21 includes
the gene that encodes for the production of the
amyloid precursor protein (APP), which in people with
Alzheimer’s is cut into beta-amyloid fragments that
accumulate into plaques. Having an extra copy of
chromosome 21 may increase the production of
beta-amyloid fragments in the brain.
Overall, people with Down syndrome develop
Alzheimer’s dementia at an earlier age than people
without Down syndrome. By age 40, most people with
Down syndrome have significant levels of beta-amyloid
plaques and tau tangles in their brains.
97
According to
the National Down Syndrome Society, about 30% of
people with Down syndrome who are in their 50s, and
about 50% of those in their 60s, have Alzheimer’s
disease dementia.
98
Emerging research suggests that
Alzheimer’s brain changes in people with Down
syndrome may be even more common than these
percentages indicate.
99, 100
As with all adults, advancing age increases the likelihood
that a person with Down syndrome will exhibit
symptoms of Alzheimer’s dementia. Life expectancy of
people with Down syndrome has more than doubled in
the last 70 years, which corresponds to a growing
population of adults living with both this condition and
dementia. Dementia is the leading cause of death for
adults with Down syndrome.
101
Care for people with
Down syndrome and dementia is especially challenging
due to the intellectual, cognitive and communication
impairments associated with Down syndrome that are
present in addition to the cognitive impairments of
dementia. Making advances in the care of people living
with Down syndrome and dementia is stymied by the
common exclusion of people with Down syndrome from
research studies.
Genetic Mutations
An estimated 1% or less of people living with Alzheimer’s
dementia develop the disease as a result of mutations to
any of three specific genes.
102
(A genetic mutation is an
abnormal change in the sequence of chemical pairs that
make up genes.) This is called dominantly inherited or
autosomal dominant Alzheimer’s dementia. These
mutations involve the amyloid protein precursor gene
and the genes for the presenilin 1 and presenilin 2
proteins. Symptoms tend to develop before age 65 and
sometimes as young as age 30. Because of this,
individuals with these mutations may also be referred to
as having younger-onset Alzheimer’s dementia. People
who inherit an Alzheimer’s mutation to these genes are
virtually guaranteed to develop the disease if they live a
normal life span.
103
However, rare cases of individuals
who have one of these mutations and do not develop
dementia symptoms until late life have recently been
reported.
104, 105
The experiences of these individuals
highlight the possibility of being resilient to Alzheimer’s
dementia despite genetic mutations, and point to new
areas of investigation to better understand resilience.
Family History
A family history of Alzheimer’s dementia is not necessary
for an individual to develop the disease. However,
individuals who have or had a parent or sibling (first-
degree relative) with Alzheimer’s dementia are more likely
to develop the disease than those who do not have a
first-degree relative with Alzheimer’s dementia.
74, 81
Those
who have more than one first-degree relative with
Alzheimer’s dementia are at even higher risk.
77
A large,
population-based study found that having a parent with
dementia increases risk independent of known genetic
risk factors such as APOE-e4.
106
When diseases run in
families, heredity (genetics) and shared non-genetic
factors (for example, access to healthy foods and habits
related to physical activity) may play a role.
Modifiable Risk Factors
Although age, genetics and family history cannot be
changed, some risk factors can be changed or modified
to reduce the risk of cognitive decline and dementia.
Examples of modifiable risk factors are physical activity,
smoking, education, staying socially and mentally active,
blood pressure and diet. In fact, The Lancet Commission
report on dementia prevention, intervention and care
suggests that up to 40% of dementia cases may be
attributable to modifiable risk factors.
107
A 2022 study
found that nearly 37% of cases of dementia in the United
States were associated with eight modifiable risk factors,
the most common being midlife obesity, followed by
physical inactivity and low educational attainment.
108
Overview
17
In addition to The Lancet Commission report, a number
of other influential reports point to the promising role
of addressing these factors to reduce risk of dementia
and cognitive decline. These include the 2019 World
Health Organization (WHO) recommendations to reduce
risk of cognitive decline and dementia and a report from
the National Academy of Medicine.
109, 110
There are many
potentially modifiable risk factors for Alzheimer’s disease
— too many to discuss in a single report. This section
focuses on some of the modifiable risk factors with
substantial supportive evidence identified in The Lancet
Commission report, the WHO recommendations and
the National Academy of Medicine report.
As mentioned earlier, most people living with dementia
have the brain changes of Alzheimer’s disease as well
as another form of dementia (see mixed dementia,
page 12), and it can be difficult to tell which brain changes
are the cause of dementia. As a result, research linking
risk factors to dementia is often assumed to support a link
between risk factors and Alzheimer’s disease. However,
additional research is needed to disentangle risk factors
that are specific to Alzheimer’s disease from those that
are specific to other causes of dementia.
111
Cardiovascular Health Factors
Brain health is affected by the health of the heart and
blood vessels. Although the brain makes up just 2% of
body weight, it consumes 20% of the body’s oxygen and
energy supplies.
112
A healthy heart ensures that enough
blood is pumped to the brain, while healthy blood vessels
enable the oxygen- and nutrient-rich blood to reach the
brain so it can function normally. One of the clearest
examples of this relationship is how stroke, which occurs
when a blood vessel in the brain is blocked or bursts,
markedly increases dementia risk.
113
Many factors that increase the risk of cardiovascular
disease are also associated with a higher risk of
dementia.
114
These factors include hypertension,
91, 115-117
diabetes
118-120
and smoking.
121, 122
Likewise, factors that
decrease risk of cardiovascular disease are associated
with decreased risk of dementia. Physical activity is an
example of a modifiable factor that reduces risk of
cardiovascular disease and may also reduce risk of
dementia.
123-133
Although researchers have studied a
wide variety of physical activities, they do not know if
specific types of physical activity are more effective at
decreasing risk, or how the frequency or duration of
physical activity may influence the effectiveness of
physical activity.
In addition to physical activity, many but not all studies
suggest that consuming a heart-healthy diet may be
associated with reduced dementia risk.
134-142
A heart-
healthy diet emphasizes fruits, vegetables, whole grains,
fish, chicken, nuts, legumes and healthy fats such as olive
oil while limiting saturated fats, red meat and sugar.
Examples of heart-healthy diets include but are not
limited to the Mediterranean, DASH (Dietary Approaches
to Stop Hypertension) and MIND (Mediterranean-DASH
Intervention for Neurodegenerative Delay) diets.
143-145
However, a recent trial of the MIND diet did not show a
difference in cognitive change for people following the
diet compared with a control group over three years.
141
It’s possible that dietary changes take many years to
influence dementia risk. No single food, beverage,
ingredient, vitamin or supplement has been proven to
prevent or cure Alzheimer's or any other dementia.
146
The risk of developing dementia in later life can be
influenced by health factors present years and
decades earlier. For example, midlife obesity,
115, 147, 148
hypertension
91, 115-117
and high cholesterol
149
are among
the midlife factors associated with an increased risk of
dementia in later life.
Today, researchers are looking at potential risk
factors present even earlier in life, such as in young
adulthood, to understand how health factors
experienced throughout one’s life span may affect later
life cognitive health.
150-154
This life course approach
offers the potential to inform preventive measures
across multiple stages of life.
Education
Researchers have long reported that people with
more years of formal education are at lower risk for
Alzheimer’s and other dementias than those with fewer
years of formal education.
86, 155-160
Much of the research
linking formal education to decreased risk of Alzheimer’s
dementia was conducted without the benefit of
technological advances such as positron emission
tomography (PET) imaging of the brain that might shed
light on whether education affects Alzheimer’s
biomarkers such as beta-amyloid and tau accumulation
that lead to dementia symptoms. More recent research
incorporating these technological advances suggests
that rather than reducing the risk of developing
Alzheimer’s brain changes, formal education may help
sustain cognitive function in mid- and late life and delay
the development of symptoms.
161, 162
18 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
To that point, some researchers believe that having
more years of education builds “cognitive reserve.”
Cognitive reserve refers to the brain’s ability to make
flexible and efficient use of cognitive networks
(networks of neuron-to-neuron connections) to
enable a person to continue to carry out cognitive
tasks despite brain changes.
163, 164
The number of years
of formal education is not the only determinant of
cognitive reserve. Having a mentally stimulating job
and engaging in other mentally stimulating activities
may also help build cognitive reserve.
165-170
Other researchers emphasize the indirect effects of
the number of years of formal education, such as its
effects on dementia risk through socioeconomic status
(SES). SES typically is defined as access to economic
resources, including income, education, employment
and occupation, but also includes factors such as
financial security and perceived social standing. Having
fewer years of formal education is associated with
lower median income and lower SES.
171
SES has many
effects on one’s health that are relevant to dementia
risk. Researchers report that lower SES is associated
with being less physically active,
172
having a higher
risk of diabetes,
173-175
and being more likely to have
hypertension
176
and to smoke
177
— all of which are risk
factors for dementia. In fact, in 2022 researchers
reported that SES is associated with changes in brain
anatomy, including gray matter volume, that may affect
overall cognitive ability.
178
In addition, lower SES may decrease one’s access to
and ability to afford heart-healthy foods that support
brain health; decrease one’s ability to afford health
care or medical treatments, such as treatments for
cardiovascular risk factors that are closely linked to
brain health; and limit one’s access to physically safe
housing and employment. Housing and employment
conditions can also influence brain health–promoting
activities and health care, as well as influence one’s
exposure to substances that are toxic to the nervous
system such as air pollution,
179
lead
180
and pesticides.
181
It’s important to realize that SES is not a biological
entity, but rather a social construct reflecting
inequities in how individuals and populations are
treated and have been treated over time. It also
reflects inequities in the perceived social standing of
individuals and populations based on factors largely
outside of their control.
Social and Cognitive Engagement
Additional studies suggest that remaining socially and
cognitively active throughout life may support brain
health and possibly reduce the risk of Alzheimer’s and
other dementias.
123, 182-190
Socially and cognitively
stimulating activity might help build cognitive reserve.
However, it is also possible that undetected cognitive
impairment decreases one’s interest in and ability to
participate in activities involving social and cognitive
skills. In this case, the association may reflect the effect
of cognitive impairment on social and cognitive
engagement rather than the effect of engagement on
dementia risk.
188
More research is needed to better
understand the mechanisms that link social and cognitive
engagement to dementia risk, along with types of
activities that provide benefit.
Traumatic Brain Injury (TBI)
TBI is a head injury caused by an external force that
results in disruption of normal brain function.
191
TBI is
associated with an increased risk of dementia.
192-194
According to the Centers for Disease Control and
Prevention (CDC), in 2020, people age 75 and older
had the highest numbers and rates of TBI-related
hospitalizations and deaths, accounting for about
32% of TBI-related hospitalizations and 28% of TBI-
related deaths.
195
In 2018 and 2019, falls were the
leading cause of TBI-related deaths among those 75
and older.
191
Two ways to classify the severity of TBI are by the
duration of loss of consciousness or post-traumatic
amnesia
196
and by the individual’s initial score on the
15-point Glasgow Coma Scale.
197
• Mild TBI (also known as a concussion) is characterized
by loss of consciousness or post-traumatic amnesia
lasting 30 minutes or less, or an initial Glasgow score
of 13 to 15; about 75% of TBIs are mild.
198
• Moderate TBI is characterized by loss of
consciousness or post-traumatic amnesia lasting
more than 30 minutes but less than 24 hours, or an
initial Glasgow score of 9 to 12.
• Severe TBI is characterized by loss of consciousness
or post-traumatic amnesia lasting 24 hours or more,
or an initial Glasgow score of 8 or less.
Moderate and severe TBIs increase risk of dementia
between 2- and 4-fold compared with risk among
individuals without a history of moderate or severe
TBI.
199
In this case, the cause of dementia is almost
always brain damage attributable to the TBI, not
Alzheimer’s disease. The risk of dementia increases with
the number of TBIs sustained.
192-194
In addition, studies
have found that people with a history of TBI who
develop dementia do so at a younger age than those
without a history of TBI.
200, 201
Whether TBI causes
dementia, other conditions that lead to dementia, or
both, is being investigated.

The relationship between TBI and chronic traumatic
encephalopathy (CTE) is a growing area of research. Like
Alzheimer’s disease, CTE is characterized by tangles of an
abnormal form of the protein tau in the brain. Beta-
amyloid plaques may also be present, with one study
indicating that more than 50% of individuals with CTE had
beta-amyloid plaques.
202, 203
The brain changes of CTE can
only be identified at autopsy. The greatest risk factor for
developing CTE-related brain changes is repetitive brain
trauma — repeated, forceful blows to the head that do
not, individually, result in symptoms.
204
Among former
amateur and professional football players, the odds of
developing CTE increased 30% per year played.
205
A review
of published articles examining CTE suggests that the
relationship between these repeated impacts and
CTE is likely causal.
206
Other Risk Factors
As mentioned earlier, there are many potentially
modifiable risk factors for dementia. Among those with
growing supportive evidence are the following.
Sleep
Among the many factors being studied is inadequate
sleep or poor sleep quality.
207-209
Researchers have found
that an important function of sleep is the removal of
toxic beta-amyloid and other substances from the
brain.
210, 211
Inadequate or poor sleep may compromise
the brain’s ability to remove beta-amyloid and other
toxins, enabling levels of toxins to remain elevated. In
addition, poor sleep quality such as that caused by
obstructive sleep apnea may increase risk by interfering
with blood flow to the brain and normal patterns of
brain activity that promote memory and attention.
212, 213
As discussed earlier, many researchers believe that the
relationship between sleep and Alzheimer’s disease is
bidirectional, meaning that not only may poor sleep
increase one’s risk of Alzheimer’s, but also that the brain
changes of Alzheimer’s may increase the risk of poor
sleep.
214-216
For example, increases in beta-amyloid and
tau may interrupt the sleep-wake cycle,
217
leading to
increased sleep fragmentation and wakefulness and
decreased slow-wave sleep.
63
Poor sleep may have
similar bidirectional relationships with other causes of
dementia, including poor cerebrovascular health.
218
Air Pollution
There is also rapidly emerging evidence on how
exposure to toxicants in the environment, especially air
pollution, may be related to dementia risk. A number of
different air pollutants have been studied in relation to
cognition, cognitive decline and dementia itself. The
most consistent and rigorous results concern fine
particulate matter (PM) air pollution. PM consists of tiny
solid particles and liquid droplets generated by fuel
combustion, fires and processes that produce dust.
PM
2.5
, particulate matter that is 2.5 microns in diameter
or smaller, is small enough to be inhaled deeply into the
lungs. This subset of PM particles has been shown to
have the greatest health impact and is the focus in most
studies. Based on its sweeping review in 2019, the U.S.
Environmental Protection Agency judged long-term
exposure to PM
2.5
as “likely to be causal” in relation to
“nervous system effects.”
219
Studies specific to dementia
and related outcomes have reported that higher
long-term exposure to PM
2.5
is associated with worse
cognitive decline,
179, 220
reduced brain volumes
179
and
increased rates of incident (newly onset) dementia.
220-222
Whether air pollution promotes the brain changes of
Alzheimer’s or other dementias is unclear.
Critical illness in older adults
A growing body of evidence suggests that critical illness
and medical encounters such as hospitalization in older
people increase their risk of long-term cognitive
impairment and dementia.
223-227
There are a number of
ways that critical illness and aspects of the hospital
experience may affect the brain.
228
One example is that
experiencing hospitalization may make older adults more
vulnerable to the existing brain changes of dementia.
229
This is not to suggest that hospitalization should be
avoided if one is ill; rather, researchers are focusing on
specific aspects of hospitalization, such as prolonged
sedation, immobilization, and lack of family engagement
that may increase risk of cognitive impairment.
228
Furthermore, experiencing delirium — a sudden and
transient state of confusion common in hospitalized
older adults — has been linked to long-term cognitive
decline and dementia.
228,230
Modifying these aspects
of hospitalization may decrease risk of cognitive decline.
In addition, better preventive health measures and
improved and expanded health care coordination may
help to prevent critical illness and subsequent
hospitalization and the negative cognitive outcomes
that may follow.
Additional research is needed to build the evidence for
these and other risk factors being investigated and,
importantly, to determine how such risk factors may
vary for different causes of dementia, across the
lifecourse, and among different racial and ethnic groups.
Overview
19

20 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Looking to the Future
Importance of Biomarkers
The discovery that Alzheimer’s disease begins 20 years
or more before the onset of symptoms suggests that
there is a substantial window of time in which we may
be able to intervene in the progression of the disease.
Scientific advances are already helping the field to make
progress in these presymptomatic years. For example,
advances in the identification of biomarkers for
Alzheimer’s disease make it possible to identify
individuals who have beta-amyloid accumulation in
the brain and who may qualify for clinical trials of
experimental treatments that aim to reduce the
accumulated beta-amyloid and in doing so prevent or
delay the onset of symptoms. Biomarkers also enable
earlier detection of the brain changes of Alzheimer’s
disease, giving those affected the opportunity to address
modifiable risk factors that may slow or delay cognitive
decline. Biomarkers are already accelerating the
development of new treatments by making it possible for
clinical trials to specifically recruit individuals with the
brain changes that experimental therapies target. In
addition, biomarker, basic science and other research
advances offer the potential to expand the field’s
understanding of which therapies or combination of
therapies may be most effective at which points in the
Alzheimer's disease continuum.
When validated biomarker tests become available for
routine use in health care providers’ offices and other
clinical settings, it will be important to provide
educational materials to help individuals and their
families understand the risks and benefits of biomarker
tests, make informed decisions about whether to have
biomarker testing, and know what to expect in care after
testing.
231, 232
On a broader scale, biomarker disclosure
may have social and societal implications. For example,
biomarker results that are positive for increased
dementia risk and that are shared with others may result
in individuals experiencing the social stigma and
discrimination so often experienced by people living with
dementia, even though individuals with increased risk may
never develop dementia.
233
In addition, disclosure may well
highlight the need for reform in societal areas such as
health insurance coverage and costs, the capacity of the
health care workforce, and health equity.
233
The Need for Increased Diversity in Research
Participation
A fuller understanding of Alzheimer’s — from its causes
to how to prevent, manage and treat it — depends on
crucial factors outside of biomarker, basic science and
other research advances. Among these is the inclusion
of participants from diverse racial and ethnic groups in
research. The lack of inclusion has several consequences.
First, without adequate data from diverse racial and
ethnic groups, the current and future burden of
Alzheimer’s disease and Alzheimer’s dementia in the
United States cannot be accurately measured.
234
Such
data is necessary because the populations of older adults
from these groups make up nearly a quarter of the
nation’s older adult population, and that share is
projected to grow.
235
Second, current data indicate that,
compared with non-Hispanic White older adults, Black
and Hispanic older adults are at increased risk for
Alzheimer’s dementia (see Prevalence section).
Alzheimer’s research with too few Black and Hispanic
participants to reflect the proportion of these groups in
the overall population largely ignores populations who
bear the greatest risk. As a result, risk factors common
in these populations but less common in non-Hispanic
White older adults are likely to be poorly understood. In
addition, lack of inclusion limits our ability to understand
whether and how dementia risk factors and
interventions work in populations that carry different
baseline susceptibility to Alzheimer’s disease including
those with Down syndrome.
Inclusion is more than a matter of enrolling more
participants from underrepresented groups. Increasing
diversity among researchers and engaging with and
seeking input from marginalized communities are also
important. Improving inclusion in all of these ways
expands the range of lived experiences among
participants and the extent to which those experiences
are known and become topics of investigation.
236
Only
by improving representation in the participation and
leadership of clinical trials, observational studies and
other investigations will everyone have the potential to
benefit from advances in dementia research.

21
Prevalence
An estimated 6.9 million
Americans are living with
Alzheimer's dementia.
This section reports on the number and
proportion of people with Alzheimer’s
dementia to describe the magnitude of the
burden of Alzheimer’s dementia on
communities, health care systems and social
safety nets. The prevalence of Alzheimer’s
dementia refers to the number and proportion
of people in a population who have Alzheimer’s
dementia at a given point in time. Incidence
refers to the number or rate of new cases per
year, often expressed as the number of people
per 100,000 who newly develop the condition
in a year. This section reports estimates from
several studies of the number of people and
proportion of the population with Alzheimer’s
or other dementias. Those estimates vary
depending on how each study was conducted.
The number and proportion of Americans with
Alzheimer’s or other dementias is expected to continue
to grow in coming years because the risk of dementia
increases with advancing age. The population of
Americans age 65 and older is projected to grow from
58 million in 2022 to 82 million by 2050.
237
By 2030, all
members of the of the baby-boom generation (Americans
born between 1946 and 1964) will be age 65 or older,
238
the age range of greatest risk of Alzheimer’s dementia;
239
in fact, the oldest members of the baby-boom generation
turned age 75 in 2021. A number of recent studies
have reported that the incidence rate of Alzheimer’s
and other dementias appears to have declined in recent
decades (see “Trends in the Prevalence and Incidence of
Alzheimer’s Dementia Over Time” in this section). This
decline in incidence has been attributed to improvements
over the 20th century in modifiable risk factors for
dementia, such as increased prevention and treatment of
hypertension and greater educational attainment.
240
It is
Millions of Americans are living with Alzheimer’s or other
dementias. As the size of the U.S. population age 65 and older
continues to grow, so too will the number and proportion of
Americans with Alzheimer’s or other dementias.
Prevalence of Alzheimer’s and Other
Dementias in the United States
An estimated 6.9 million Americans age 65 and older
are living with Alzheimer’s dementia in 2024.
A2,241
Seventy-three percent are age 75 or older (see Figure 2,
page 23).
241
Of the total U.S. population:
• About 1 in 9 people (10.9%) age 65 and older has
Alzheimer’s dementia.
A2,241
• The percentage of people with Alzheimer’s dementia
increases with age: 5.0% of people age 65 to 74,
13.2% of people age 75 to 84, and 33.4% of people
age 85 and older have Alzheimer’s dementia.
A2, 241
People younger than 65 can also develop Alzheimer's
dementia. Although prevalence studies of younger-
onset dementia in the U.S. are limited, researchers
believe about 110 of every 100,000 people age 30 to
64 years, or about 200,000 Americans in total, have
younger-onset dementia.
242
The estimated number of people age 65 and older with
Alzheimer’s dementia comes from an updated study using
the latest data from the 2024 population projections from
the U.S. Census Bureau and the Chicago Health and Aging
Project (CHAP), a population-based study of chronic
health conditions of older people.
241
unknown how COVID-19, including infection with SARS-
CoV-2 (the virus that causes COVID-19), mortality from
COVID-19, and changes in health care access resulting
from the COVID-19 pandemic will influence the number
and proportion of people in the U.S. with Alzheimer’s
dementia in years to come. However, even with this
potentially lower incidence rate and the impact of COVID
on people at risk of dementia, the absolute number of
people with Alzheimer’s and other dementias is expected
to continue growing because of the large increase in the
number of adults age 65 and older, the age group that is
at increased risk of Alzheimer’s and many other dementias.
22 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).

23
Whereas CHAP generated estimates specific to
Alzheimer’s dementia, national estimates of the
prevalence of all dementias combined are available from
other population-based studies, including the Health
and Retirement Study (HRS), a nationally representative
sample of older adults. Based on newly available estimates
from HRS’s Harmonized Cognitive Assessment Protocol
(HCAP), 10% of people age 65 and older in the U.S. had
dementia in 2016.
A3, 160
Underdiagnosis of Alzheimer’s and Other
Dementias in the Primary Care Setting
Prevalence studies such as CHAP and HRS are designed
so that everyone in the study undergoes evaluation for
dementia. But outside of research settings, a substantial
portion of those who would meet the diagnostic criteria
for Alzheimer’s and other dementias are not diagnosed
with dementia by a physician.
252-261
Furthermore, only
about half of Medicare beneficiaries who have a diagnosis
of Alzheimer’s or another dementia in their Medicare
billing records report being told of the diagnosis.
262-266
Because dementia is often underdiagnosed — and if
it is diagnosed by a clinician, some people appear to
be unaware of their diagnosis — a large portion of
Americans with dementia may not know they have it.
267
Some studies indicate that underdiagnosis is higher in
Black and Hispanic older adults.
260, 261, 268
A number of
potential harms may result from a missed or delayed
dementia diagnosis. These include delayed access to
treatment, less time for care planning, higher costs of
care, and negative impact on the individual’s physical or
mental health or even the mental health of their family
members and potential caregivers; more research is
needed to better understand the potential harms of
delayed or lack of diagnosis.
269
Underdiagnosis is most
pronounced at the earliest stages of dementia when
symptoms are mild.
268
Even fewer people living with mild
cognitive impairment (MCI), a precursor to dementia
(see Overview page 10), receive a diagnosis despite this
being a stage where treatment and planning may be most
effective.
270
One recent study estimates that only 8% of
older Americans living with MCI receive a diagnosis.
271
Prevalence of Subjective Cognitive Decline
Subjective cognitive decline refers to an individual’s
perception that their memory or other thinking abilities
are worsening, independent of cognitive testing or a
physician’s diagnosis. Subjective cognitive decline is one
of the earliest warning signs of dementia and may be a
way to identify people who are at high risk of developing
Alzheimer’s or other dementias, as well as MCI.
272-276
Not
all those who experience subjective cognitive decline
go on to develop MCI or dementia, but many do.
277-279
Subjective cognitive decline often prompts medical
attention, and a formal diagnosis can help distinguish
experiences potentially related to higher dementia risk
from experiences less likely to be related, such as other
underlying health conditions.
280
One study showed
those who consistently reported subjective cognitive
decline that they found worrisome were at higher risk for
developing Alzheimer’s dementia.
281
The Behavioral Risk
Factor Surveillance System survey, a large cross-sectional,
telephone-based survey of community-dwelling people
across the U.S. that includes questions on subjective
cognitive decline, found that 10% of Americans age 45
and older reported subjective cognitive decline, but 54%
of those who reported it had not consulted a health care
professional.
282
Individuals concerned about declines in
memory and other cognitive abilities should consult a
health care professional.
Prevalence
*Percentages do not total 100 due to rounding.
Created from data from Rajan et al.
A2, 241
65-74 years:
1.83 million (26.4%)
75-84 years:
2.67 million (38.6%)
85+ years:
2.42 million (35.4%)
Number and Ages of People 65 or Older
with Alzheimer's Dementia, 2024*
Total:
6.9 Million
Figure
2

Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).24
Prevalence Estimates
The prevalence numbers included in this report are
based on estimates of how many people in the U.S. are
living with Alzheimer’s dementia; that is, the number
of people living with the clinical symptoms described
in the “Dementia Due to Alzheimer’s Disease” (mild,
moderate, or severe) portion of the “Alzheimer’s Disease
Continuum” described on pages 10-11 of the Overview.
The estimate of 6.9 million older adults who have
Alzheimer’s dementia comes from a single longitudinal
study in which participants were systematically evaluated
and then re-evaluated on a regular basis; those who
exhibited the clinical symptoms of Alzheimer’s dementia
were classified as having Alzheimer’s dementia.
A2, 241
A major advantage of this approach is that it attempts to
capture all individuals living with the condition and does
not rely on the diagnosis of people living with Alzheimer’s
by the health care system, a process that has resulted
in a substantial undercount (i.e., “underdiagnosis”) of
the Alzheimer’s population. The disadvantage is that the
longitudinal study is located in a single, small geographic
area and may not be nationally representative (although
the estimation process attempted to account for
the demographics of the entire U.S. population). In
the future, Facts and Figures could report estimates
of Alzheimer’s dementia prevalence from multiple
longitudinal studies or using different symptom-based
diagnostic criteria; these differences in criteria could
result in different prevalence estimates from what we
report here.
A3, 160
Almost all existing Alzheimer’s dementia prevalence
studies are based on the identification of clinical
symptoms to classify an individual as having Alzheimer’s
dementia; they do not rely on the brain changes believed
to be responsible for Alzheimer’s disease across the
continuum of the disease. As data sources, methods and
scientific knowledge improve, estimates of prevalence may
incorporate these brain changes using biomarkers. This
addition could lead to very different prevalence estimates
for a number of reasons, which are discussed below.
Prevalence Estimates of Dementia Due to Alzheimer’s
Disease Based on Biomarkers and Dementia Symptoms
Prevalence estimates of dementia due to Alzheimer’s
disease based on Alzheimer’s brain changes, as well
as overt clinical dementia symptoms, are likely to be
lower than the 6.9 million figure reported here. This
is because autopsy- and biomarker-based studies
21, 79,
243-245
indicate that some individuals counted as having
Alzheimer’s dementia based on symptoms do not have
the biological brain changes defined as Alzheimer’s
disease; that is, their dementia is caused by something
other than Alzheimer’s disease. Both autopsy studies and
clinical trials have found that 15% to 30% of individuals
who meet the criteria for clinical Alzheimer’s dementia
based on symptoms did not have Alzheimer’s-related
brain changes. Thus, these studies indicate that estimates
using biomarkers of Alzheimer’s disease could be up to
30% lower than prevalence estimates based only on
symptoms. This would translate to roughly 4.8 million
Americans age 65 and older being classified as having
dementia due to Alzheimer’s disease in 2024.
A3, 160
Prevalence Estimates of MCI due to Alzheimer’s Disease
Based on Biomarkers and Mild Cognitive Symptoms
For decades it has been recognized that all individuals
with dementia pass through a precursor stage
frequently referred to as mild cognitive impairment
(MCI; see Overview, page 10). With the recent advent
of biomarkers that detect the brain changes believed
to characterize Alzheimer’s disease, it may now be
possible to determine which individuals diagnosed with
MCI have MCI due to Alzheimer’s disease. The number
and proportion of older adults who have MCI due to
Alzheimer’s disease are currently difficult to estimate
because they require studies with both population-based
prevalence measures of MCI and tests of Alzheimer’s
biomarkers, and this line of research is in its infancy.
Furthermore, there is variation across studies in both
the threshold of cognitive impairment required for an
MCI diagnosis and the level of biomarker burden that
defines the presence of Alzheimer’s disease. However,
we can roughly estimate this prevalence indirectly using
multiple data sources. A systematic review of more than
30 studies of all-cause MCI reported that about 17%
of people age 65 and older had MCI.
51
The HRS HCAP
study more recently estimated the prevalence of MCI in
people age 65 and older to be 22%.
160
Meanwhile, studies
assessing biomarkers for Alzheimer’s disease with PET
scans have reported that about half of people with MCI
have Alzheimer’s-related brain changes.
246, 247
Therefore,
roughly 8% to 11% of the 63 million Americans who
are age 65 and older in 2024 — or approximately
5 to 7 million older Americans — may have MCI due to
Alzheimer’s disease.
248
This broad prevalence estimate
needs to be refined with population-based studies
involving biomarkers and more precise estimates from
narrower age ranges.

25
Prevalence Estimates of Alzheimer’s Disease
Based on Biomarkers and any Cognitive Symptoms
(MCI or Dementia)
Combining the estimates of the prevalence of dementia
due to Alzheimer’s disease and the prevalence of MCI
due to Alzheimer’s disease provides an estimate of
people living with the brain changes of Alzheimer’s
disease and some form of cognitive impairment. This
estimate would include older adults with the earliest
detectable stages of cognitive impairment who have
the brain changes of Alzheimer’s but may or may not
have the overt symptoms of dementia that interfere
with their ability to carry out everyday activities.
Combining the estimates of roughly 4.8 million
Americans age 65 and older with dementia due to
Alzheimer’s disease based on Alzheimer’s brain changes
and the 5 to 7 million older Americans with MCI due
to Alzheimer’s disease translates to approximately
10 to 12 million older Americans with Alzheimer’s
disease and some form of cognitive impairment in 2024.
Furthermore, because MCI develops years before
dementia onset and can affect individuals younger
than 65, there are likely more than 5 to 7 million
people of any age with MCI due to Alzheimer’s disease,
and thus the 10 to 12 million estimate could be even
higher if we consider Americans of all ages, not just
those 65 or older.
Prevalence Estimates of Alzheimer’s Disease
Across the Entire Cognitive Spectrum
Finally, as measurements of the brain changes of
Alzheimer’s disease become more widely available in
research, we will be able to estimate how many people
have Alzheimer’s disease regardless of the presence
or absence of dementia or any form of cognitive
impairment. The total number of people living with the
brain changes of Alzheimer’s disease is likely to be much
larger than the number with MCI or dementia due to
Alzheimer’s disease given that there is an incipient and
silent (i.e., “preclinical”) stage of Alzheimer’s disease
before the emergence of cognitive symptoms of either
MCI or dementia (see Overview, page 10).
249
While this
is still the subject of ongoing research, estimates are
emerging of the prevalence of preclinical Alzheimer’s
disease in the population.
250, 251
More research is needed
to validate preclinical Alzheimer's and determine how to
measure it with biomarkers that conclusively represent
Alzheimer’s disease, as opposed to other dementia-
Prevalence
causing diseases. We also need to further understand
if this preclinical stage is a valid representation of
people who may go on to develop dementia due to
Alzheimer’s disease. When a conclusive connection is
shown between biomarkers and the preclinical stage,
and when epidemiological studies include biomarker-
based diagnoses, it will be possible to estimate the
number of individuals throughout the entire continuum
of Alzheimer’s disease (i.e., those with biomarker-
confirmed Alzheimer’s dementia, those with biomarker-
confirmed MCI due to Alzheimer’s disease and those with
biomarker-confirmed preclinical Alzheimer’s disease).
The resulting estimated prevalence will be even higher
than any estimates presented in the current report.
Future Facts and Figures Prevalence Estimates
What does all this mean for future prevalence estimates?
Future Facts and Figures reports will continue to include
the estimated prevalence of individuals in the Alzheimer’s
dementia stage, defined according to clinical symptoms,
currently estimated at 6.9 million Americans, in addition
to the best available estimated prevalence of MCI due
to Alzheimer’s disease. Accurate, up-to-date estimates
of the number of people living with these conditions
will remain essential to understanding the demands
on affected families, health systems, social and health
safety nets, and, of course, the people living with these
conditions. When biomarker-based prevalence estimates
become available, Facts and Figures will also report the
estimated prevalence of individuals with any clinical
cognitive impairment and Alzheimer’s disease to reflect
both those in the dementia phase and those in the MCI
phase of Alzheimer’s. Facts and Figures will not include
prevalence estimates of the preclinical Alzheimer’s
disease stage until (1) there is convincing evidence of a
connection between biomarkers in this silent stage
and the development of MCI due to Alzheimer’s disease
and (2) epidemiologic studies have estimated the number
of individuals in this stage. In addition, as the evidence
and epidemiological data warrant, future reports may
also include estimates of the prevalence of dementia
from all causes. It should be noted that both symptom-
based prevalence estimates of Alzheimer’s dementia
and biomarker-based prevalence estimates of
Alzheimer’s disease are expected to increase in the
future due to growth in the population of Americans
age 65 and older, the group most at risk for developing
cognitive symptoms.

26 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
State
Number
(in thousands)
%
Montana 21 9.8
Nebraska 35.1 11
Nevada 54.9 10.6
New Hampshire 26.5 10.1
New Jersey 185.3 12.3
New Mexico 46 11.8
New York 426.5 12.7
North Carolina 210.5 11.6
North Dakota 13.7 11.1
Ohio 236.2 11.3
Oklahoma 70.5 10.8
Oregon 79.1 10
Pennsylvania 282.1 11.5
Rhode Island 22 11.4
South Carolina 112.5 11.5
South Dakota 16.5 10.5
Tennessee 129.2 10.9
Texas 459.3 11.9
Utah 38.3 10
Vermont 12.8 9.9
Virginia 164 11.7
Washington 126.7 10.2
West Virginia 38.1 10.2
Wisconsin 110.9 10.6
Wyoming 10.3 9.9
Estimated Prevalence (Number and Percentage) of Alzheimer’s Dementia (AD) in the 50 U.S. States
and District of Columbia Among Adults Age 65 Years and Older in 2020
State
Number
(in thousands)
%
Alabama 103.6 11.8
Alaska 8.4 8.8
Arizona 151.5 11
Arkansas 60.4 11.3
California 719.7 12
Colorado 90.8 10.4
Connecticut 76.8 11.9
Delaware 22.3 11.3
District of Columbia 15.1 16.8
Florida 579.9 12.5
Georgia 188.3 12
Hawaii 31.2 11.3
Idaho 29.9 9.8
Illinois 250.6 12
Indiana 121.3 10.9
Iowa 62.1 11
Kansas 54.5 11.2
Kentucky 80.5 10.4
Louisiana 94.7 12.4
Maine 29.6 10.1
Maryland 127.2 12.9
Massachusetts 135.2 11.3
Michigan 202.8 11.2
Minnesota 101.9 10.7
Mississippi 62.5 12.5
Missouri 122.3 11.2
Created from data from Dhana et al.
283
Table
4

27
* Only counties with 10,000 or more residents age 65 or older were included in the ranking.
Created from data from Dhana et al.
283
Prevalence
8.8% - 9.9% 10.0% - 10.9% 11.0% - 11.9% 12.0% - 12.9%
Prevalence of Alzheimer's Disease in the 50 U.S. States, and the 10 Counties with the Highest Prevalence, 2020*
4 Prince George’s County, MD
(16.1%)
5 Hinds County, MS
(15.5%)
6 Orleans Parish, LA
(15.4%)
7 Dougherty County, GA
(15.3%)
10
9
8
7
1 Miami-Dade County, FL
(16.6%)
2 Baltimore City, MD
(16.6%)
3 Bronx County, NY
(16.6%)
1
2
4
3
5
6
8 Orangeburg County, SC
(15.2%)
9 Imperial County, CA
(15.0%)
10 El Paso County, TX
(15.0%)
Figure
3

28 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Estimates of the Prevalence of Alzheimer’s
Dementia by State and County
Recently, an analysis was conducted using the same data
sources that generated the national prevalence estimate
in this report that provides estimates of the prevalence
of Alzheimer’s dementia by state and, for the first time,
by county.
283
As shown in Figure 3, states and counties in
the eastern and southeastern U.S. have the highest
prevalence of Alzheimer’s dementia; eight of the
10 counties (with at least 10,000 older adults) with the
highest prevalence are in the East and Southeast. In
these regions, older people and Black and Hispanic
residents — groups that are at higher risk of Alzheimer's
dementia — comprise larger percentages of the
population (see Racial and Ethnic Differences in the
Prevalence of Alzheimer’s and Other Dementias on pages
29-31). Table 4 displays the prevalence (both number
and percentage)of Alzheimer’s dementia for each state.
Understanding these regional differences can help guide
the allocation of resources to public health programs for
Alzheimer’s in the U.S.
Incidence of Alzheimer’s Dementia
While prevalence refers to existing cases of a disease in a
population at a given time, incidence refers to new cases
of a disease that develop in a given period in a defined
population — for example, the number of people who
develop Alzheimer’s dementia during 2024 among U.S.
adults who are age 65 or older. Incidence provides a
measure of risk for developing a disease. According to
estimates using data from the CHAP study and the U.S.
Census Bureau, approximately 910,000 people age 65 or
older developed Alzheimer’s dementia in the U.S. in 2011,
a number that would be expected to be even higher in
2024 if CHAP estimates were available for that year.
284
The rate at which new cases of Alzheimer’s develop
increases dramatically with age: according to estimates
from CHAP, in 2011 the average annual incidence in
people age 65 to 74 was 0.4% (meaning four of every
1,000 people age 65 to 74 developed Alzheimer’s
dementia in 2011); in people age 75 to 84, the annual
incidence was 3.2% (32 of every 1,000 people); and in
people age 85 and older, the incidence was 7.6% (76 of
every 1,000 people).
284
A 2015 study using data from the
Adult Changes in Thought Study, a cohort of members of
a health care delivery system in the Seattle area, reported
similar incidence rates to the CHAP study.
10
Because of
the increasing number of people age 65 and older in the
U.S., particularly those age 85 and older, the annual
number of new cases of Alzheimer’s and other dementias
is projected to double by 2050.
285
Lifetime Risk of Alzheimer’s Dementia
Lifetime risk is the probability that someone of a given
age who does not have a particular condition will develop
the condition during that person’s remaining life span.
Data through 2009 from the Framingham Heart Study
were used to estimate lifetime risk of Alzheimer’s
dementia by age and sex.
A4, 286
As shown in Figure 4, the
study estimated that the lifetime risk for Alzheimer’s
dementia at age 45 was approximately 1 in 5 (20%) for
women and 1 in 10 (10%) for men. The risks for both
sexes were slightly higher at age 65.
286
Differences Between Women and Men in
the Prevalence and Risk of Alzheimer’s and
Other Dementias
Almost two-thirds of Americans with Alzheimer’s are
women.
241
Of the 6.9 million people age 65 and
older with Alzheimer’s dementia in the United States,
4.2 million are women and 2.7 million are men.
241
This
represents 11% of women and 9% of men age 65 and
older in the United States.
287
Women live longer than men on average, and older
age is the greatest risk factor for Alzheimer’s.
286, 288, 289
This survival difference contributes to higher prevalence
of Alzheimer’s and other dementias in women compared
with men. However, it is not clear that the risk of
developing Alzheimer’s or other dementias differs
between men and women of the same age. Most studies
of incidence in the United States have found no
meaningful difference between men and women in the
proportion who develop Alzheimer’s or other dementias
Created from data from Chene et al.
286
10.3%
19.5%
25
20
15
10
5
0
45Age 65
11.6%
21.1%
MenPercentage Women
Estimated Lifetime Risk for Alzheimer’s Dementia,
by Sex, at Ages 45 and 65
Figure
4
29
at any given age,
10, 86, 289-291
while some European studies
have reported a higher incidence among women at older
ages,
292, 293
or higher incidence among men.
294
Therefore,
differences in the risk of dementia between men and
women may depend in part on age, birth cohort and/or
geographic region.
295, 296
Other studies have provided evidence that any observed
difference in dementia risk between men and women may
be an artifact of who is more or less likely to die of other
health factors before developing dementia. A study using
Framingham Heart Study data suggested that men in the
study appeared to have a lower risk for dementia due to
“survival bias,” in which the men who survived to age 65
or beyond and were included in the study were the ones
with a healthier cardiovascular risk profile (men have a
higher rate of death from cardiovascular disease in middle
age than women) and thus a lower risk for dementia.
288
Recent studies have supported the notion that selection
bias contributes to reports of sex and gender differences
in Alzheimer’s dementia risk.
285, 297
More research is
needed to support this interpretation.
Although differences in the rates at which men and
women develop Alzheimer’s or other dementias do not
appear to be large or consistent, the reasons men and
women develop dementia may vary. These differences
may be based in biology, such as chromosomal or
hormonal differences related to reproductive history
298
(i.e., sex differences), or in how social and cultural factors
are distributed among or are experienced by men and
women (i.e., gender differences), or a combination of the
two.
295, 299, 300
Gender differences may exist in the
distribution of or even the effect of known risk factors for
dementia, such as education, occupation, cardiovascular
disease and health behaviors. For example, lower
educational attainment in women than in men born in the
first half of the 20th century may contribute to elevated
risk in women, as limited formal education is a risk factor
for dementia.
301
This possibility requires more research,
but evidence supports that greater educational
attainment over time in the United States — the gains in
which have been more substantial for women than men
— has led to decreased risk for dementia.
302
In addition to
differences in educational attainment relating to
dementia risk differences in men and women, the same
level of education affect men's and women's dementia risk
differently. European studies have found that the
association of lower educational attainment with
dementia outcomes may be stronger in women than
men.
303, 304
Other societal gender differences may also be at play,
such as differences in occupational attainment between
men and women, with a recent study showing that women
who participated in the paid workforce earlier in life had
better cognitive outcomes after age 60 than women
who were not part of the paid workforce.
297, 305, 306
More
recently, gender differences during the lockdown phase
in the early part of the COVID-19 pandemic included
increased child care and job loss in sectors where women
were more likely to be employed.
307-309
It is unclear how
these differential impacts on women may affect their
brain health in the future. Researchers have begun
exploring how mental health challenges, lost job
opportunities, and decreased employment earnings
experienced during the pandemic may affect women’s
ability to maintain brain health.
308
It is unclear whether genetic risk operates differently in
women and men in the development of, or susceptibility to,
Alzheimer’s pathology.
310
A number of studies have
indicated that the APOE-e4 genotype, the best known
common genetic risk factor for Alzheimer’s dementia, may
have a stronger association with Alzheimer’s dementia
311, 312
and neurodegeneration
313
in women than in men. However,
a meta-analysis found no difference between men and
women in the association between APOE-e4 and
Alzheimer’s dementia overall, although age played an
interesting interactive role. That is, APOE-e4 was related
to higher Alzheimer’s risk in women than men between
ages 55 and 70, when APOE is thought to exert its largest
effects.
314
It is unclear whether the influence of APOE-e4
may depend on the sex hormone estrogen.
315, 316
It should be recognized that sex and gender identities
cannot be reduced to binary categories. Individuals who
identify with nonbinary sex or gender identities may have
different risks for Alzheimer’s disease (see “Risk for
Alzheimer’s and Other Dementias in Sexual and Gender
Minority Groups” in this section).
Racial and Ethnic Differences in the Prevalence
and Risk of Alzheimer’s and Other Dementias
The risk of Alzheimer’s and other dementias appears to
vary by race and ethnicity in the U.S. While risk is poorly
characterized in smaller racial and ethnic groups in the
U.S., multiple studies have reported on differences in risk
across non-Hispanic Black, non-Hispanic White, and
Hispanic Americans. In the U.S., non-Hispanic Black and
Hispanic older adults are more likely than White older
adults to have Alzheimer’s or other dementias.
317-323
Data
from the CHAP study indicates 19% of Black and 14%
of Hispanic adults age 65 and older have Alzheimer’s
dementia compared with 10% of White older adults.
241
In line with these observations, most other prevalence
studies indicate that Black older adults are about twice as
likely to have Alzheimer’s or other dementias as White
older adults.
160, 284, 324, 325
Some other studies indicate
Hispanic older adults are about one and one-half times as
likely to have Alzheimer’s or other dementias as White
Prevalence
30 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
older adults,
325-327
though others have shown similar
prevalences among Hispanic older adults and White older
adults.
160
The population of Hispanic people comprises
very diverse groups with different cultural histories and
health profiles, and there is evidence that prevalence may
differ from one specific Hispanic ethnic group to another
(for example, Mexican Americans compared with
Caribbean Americans).
328, 329
The higher prevalence of Alzheimer’s dementia in Black
and Hispanic populations compared with the White
population appears to be due to a higher risk of
developing dementia in these groups compared with the
White population of the same age.
330, 331
Race is a social
construct with little to no genetic or biological basis.
Instead, race is an idea created and used throughout
history by groups in power to justify their control and
dominance over other groups, and genetic factors do not
account for the large differences in prevalence and
incidence among racial groups.
330, 332
While there is some
research into how the influence of genetic risk factors on
Alzheimer’s and other dementias may differ by race —
for example, the influence of the APOE-e4 allele on
Alzheimer’s risk may be stronger for White Americans
than Black Americans
88-92, 333
— these small differences in
genetic influence do not account for the large differences
in dementia risk across racial groups. Instead, research
suggests that the historic and continued marginalization
of Black and Hispanic people in the U.S. has produced
disparities between older Black and Hispanic populations
and older White populations in life experiences,
socioeconomic indicators and, ultimately, health
conditions. It is these disparities that most likely explain
the difference in risk for Alzheimer’s and other dementias
among racial and ethnic groups.
334
These health and
socioeconomic disparities are rooted in the history of
discrimination against Black individuals and other people
of color in the U.S., not only during interpersonal
interactions, but also as codified in the rules, practices
and policies of U.S. banks, laws, health care and other
systems — that is, structural racism.
335, 336
Structural
racism pervades many aspects of life that may directly or
indirectly alter dementia risk, including where people can
live, the quality of schools in their communities, exposure
to harmful toxicants and pollutants, access to quality
health care, employment prospects, occupational safety,
the ability to pass wealth to subsequent generations,
treatment by the legal system and exposure to
violence.
337-340
The cumulative stress imparted by the effects of
structural racism and the resulting differences in social
and physical environments may directly influence
dementia risk among historically marginalized and socially
disadvantaged racial and ethnic groups. Further, structural
racism leads to disparities by race and ethnicity in a wide
range of health outcomes including increased risk for
chronic conditions that are themselves associated with
higher dementia risk, such as cardiovascular disease and
diabetes. These health conditions, which disproportionately
affect Black and Hispanic populations, are believed to
explain much of the elevated risk of dementia among Black
and Hispanic populations.
88, 334, 341, 342
Many studies suggest
that racial and ethnic differences in dementia risk do not
persist in rigorous analyses that account for health and
socioeconomic factors.
156, 330, 343
The influence of structural racism on health and dementia
risk may cascade and compound across the course of a
person’s life. For example, some studies indicate that early
life experiences with residential and school segregation can
have detrimental effects on the cognitive health of Black
Americans in later life.
337-339
This points to a need for health
disparities researchers to employ a life course perspective
and to seek the insights of race equity scholars to account
for the cumulative interplay of many environmental and
sociopolitical factors that may put some groups of people
at increased risk for Alzheimer’s and other dementias.
334, 342
Many of the social processes that influence disparities in
the development of Alzheimer’s could also influence
whether and when a diagnosis of dementia occurs. There
is evidence that missed or delayed diagnoses of Alzheimer’s
and other dementias are more common among Black
and Hispanic older adults than among White older
adults.
254, 256, 259, 344, 345
Based on data from Medicare
beneficiaries age 65 and older, it has been estimated that
Alzheimer’s or another dementia has been diagnosed in
10.3% of White older adults, 12.2% of Hispanic older
adults and 13.8% of Black older adults.
346
Although these
percentages indicate that the dementia burden is greater
among Black and Hispanic older adults than among White
older adults, the percentages should be even higher according
to prevalence studies that detect all people who have
dementia irrespective of their use of health care systems.
Population-based cohort studies regarding the prevalence
and incidence of Alzheimer’s and other dementias in racial
and ethnic groups other than White, Black and Hispanic
populations are relatively sparse.
331
Among the few studies,
one examined electronic medical records of members of a
large health plan in California. Its findings indicated that
dementia incidence — determined by the first presence of
a dementia diagnosis in members’ medical records — was
highest among African American older adults (the term
used in the study for those who self-reported as Black or
African-American); intermediate for Latino older adults
(the term used in the study for those who self-reported as
Latino or Hispanic), American Indian and Native Alaskan
older adults, Pacific Islander older adults and White
older adults; and lowest among Asian American older
adults.
347
A follow-up study with the same cohort showed
differences across Asian American subgroups, but all

31Prevalence
subgroups studied had lower dementia incidence than the
White population.
348
A systematic review of the literature
found that Japanese Americans were the only Asian
American subgroup with reliable prevalence data, and that
they had the lowest prevalence of dementia compared with
all other ethnic groups.
328
We have limited understanding of
Alzheimer’s disease as experienced by people of Middle
Eastern and North African descent,
349
those who identify
with more than one race or ethnicity, and subgroups of origin
within racial or ethnic groups.
346
More studies, especially
those involving community-based cohorts and those that
focus on racial/ethnic groups historically not included in
Alzheimer’s research, are necessary to draw conclusions
about the prevalence of Alzheimer’s and other dementias in
different racial and ethnic groups and subgroups.
Risk for Alzheimer’s and Other Dementias
in Sexual and Gender Minority Groups
There are other groups with shared social identities
and characteristics that may experience different risks
of Alzheimer’s and other dementias. This includes
members of sexual and gender minority (SGM) groups.
SGM refers to individuals who identify as lesbian, gay,
bisexual (sexual minorities), and/or transgender or
gender nonbinary, as well as people with a gender
identity, gender expression or reproductive
development that varies from traditional, societal,
cultural or physiological norms (gender minorities).
SGM older adults may face an increased dementia
risk, through pervasive exposure to systematic
discrimination, marginalization, disadvantage and/or
exclusion from social organizations and enterprises.
Those enterprises include Alzheimer's research, and,
until recently, little has been known about the dementia
risks of people who self-identify as SGM. Although few
studies have been designed to investigate whether SGM
older adults are at greater risk for dementia than
non-SGM older adults, a growing body of preliminary
evidence suggests that this may be the case. In a study
of adults living in any of 25 U.S. states, SGM older adults
reported experiencing more cognitive problems than
non-SGM older adults.
350
Two population-based studies
found higher rates of cognitive impairment among SGM
older adults than among non-SGM older adults,
351, 352
yet a third study reported that the risks for dementia
and mild cognitive impairment were similar for people in
same-sex relationships and people in another-sex
relationships.
353
Two studies found indications of
potentially elevated dementia risk among transgender
adults. One study of Medicare beneficiaries estimated
that dementia was present among 18% of transgender
adults age 65 years and older, compared with 12%
among cisgender (not transgender) adults.
354
A second
study of adults in Florida reported that transgender
adults were more likely than cisgender adults to have a
diagnosis of Alzheimer’s and other dementias in their
electronic medical records.
355
A recent review of the evidence
found that most studies examining subjective cognitive
decline as an outcome showed higher prevalence among
SGM older adults, while those examining objective
measures of cognitive performance showed more
mixed results.
356
More research is necessary to establish whether
SGM older adults face elevated dementia risk and if
so, to understand reasons for it. Researchers have
hypothesized that stressors experienced by SGM older
adults, such as discrimination and marginalization, may
elevate their risk for Alzheimer’s and other
dementias.
300
These stressors could take a toll on the
physical and mental health of SGM older adults.
357
One study showed that SGM older adults who were
experiencing depression were more likely to report
subjective cognitive decline than SGM older adults
without depression.
358
SGM older adults experience
disparities in other health-related factors that elevate
the risk of Alzheimer’s and other dementias, including
higher rates of alcohol and tobacco use, obesity and
other cardiovascular risk factors compared with
non-SGM older adults. SGM older adults also have
lower rates of health care access and preventive health
screenings, in part due to experiencing barriers such as
discrimination and heterosexist attitudes in health care
settings.
359
Finally, the history of HIV/AIDS and its
burden of illness, mortality and social stigma has been
tied to the SGM population, particularly gay and bisexual
men and transgender people. HIV/AIDS is now a chronic
condition that can be managed successfully with
medication, and many people with HIV/AIDS survive into
older ages. In addition to any effects of this history on
aforementioned social stressors and health care access,
HIV/AIDS itself is a risk factor for dementia.
360
The
elevated prevalence of HIV/AIDS in gay and bisexual men
and transgender people puts them at higher risk for
dementia due to HIV/AIDS than non-SGM older adults.
There is increasing recognition that historically
marginalized groups — whether defined by gender,
sexual orientation, race, ethnicity or other traits — are
not monolithic when it comes to their identities and
experiences. These identities and experiences intersect,
and belonging to more than one of these groups may be
particularly consequential for health, including dementia
risk. For example, a recent study showed that
This report keeps the racial, ethnic and other
population identifiers used in source documents
when describing findings from specific studies.

32 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
transgender adults from minoritized ethnoracial groups
are more likely to report subjective cognitive decline
than other transgender adults.
361
This “intersectionality”
framework is important for developing more informative
dementia research and more effective and compassionate
dementia care in these communities.
362
It is important
that research and care efforts consider how systems of
oppression based on gender, race, ethnicity, class,
sexual orientation and HIV status may intersect and
influence dementia.
363, 364
Trends Over Time in the Prevalence and
Incidence of Alzheimer’s Dementia
A growing number of studies indicate that the
prevalence (i.e., proportion)
239, 259, 290, 345-349, 365-367
and
incidence
294, 365-374
of Alzheimer’s and other dementias
in the U.S. and other high income countries may have
declined in the past 25 years,
294, 302, 365-373, 375-379
though
results are mixed.
70, 284, 380, 381
One recent systematic
review found that incidence of dementia has decreased
worldwide over the last four decades while incidence of
Alzheimer’s dementia, specifically, has held steady, but
more research on this distinction is needed, especially in
low-income and middle-income countries.
382
Declines in
dementia risk have been attributed to increasing levels
of education and improved control of cardiovascular
risk factors.
302, 368, 371, 375, 383, 384
Such findings are
promising and suggest that identifying and reducing
risk factors for dementia may be effective — whether
interventions occur person by person (such as obtaining
treatment for one’s blood pressure) or are integrated
into the fabric of communities (such as changes in
education policies). Although these findings indicate
that a person’s risk of dementia at any given age may
be decreasing slightly, the total number of people with
Alzheimer’s or other dementias in the U.S. and other
high-income countries is expected to continue to
increase dramatically because of the increase in the
number of people at the oldest ages.
It is unclear whether these encouraging declines
in incidence will continue. For example, worldwide
increases in diabetes and obesity, which are risk
factors for dementia, among people younger than
65 may lead to a rebound in dementia risk in coming
years.
366, 385-388
It is also not clear that the encouraging
trends pertain to all racial and ethnic groups.
284, 323, 383,
384, 389, 390
Thus, while recent findings are promising, the
social and economic burden of Alzheimer’s and other
dementias will continue to grow. Moreover, 68% of
the projected increase in the global prevalence and
burden of dementia by 2050 will take place in low- and
middle-income countries, where current evidence
does not support a decline in the risk of Alzheimer’s
and other dementias.
391
Finally, it is not known how
Created from data from Rajan et al.
A5,241
14
12
10
8
6
4
2
0
2020Year 2030 2040 2050 2060
Ages 65-74 Ages 75-84 Ages 85+
Millions of people
6.1
8.5
11.2
12.7
13.8
Projected Number of People Age 65 and Older (Total and by Age) in the U.S. Population
with Alzheimer’s Dementia, 2020 to 2060
Figure
5
COVID-19 will influence the prevalence and incidence
of Alzheimer’s dementia. For example, the neurologic
effects of COVID-19
392
and the pandemic’s disruptions
to general and brain-related health care may increase
the incidence of Alzheimer’s and other dementias.
Some researchers have surmised that factors such
as social isolation from lockdowns, no-visitor policies
in long-term care facilities, and increased intensive
hospitalizations may increase dementia risk at the
population level, but research in coming years will be
necessary to confirm this and examine whether the
impact is time-limited or long term. On the other
hand, the number of people living with Alzheimer’s
dementia could be influenced in the opposite direction
by increased mortality due to COVID-19 and other
causes of death during the pandemic in 2020–2023,
which may have resulted in death prior to the onset of
Alzheimer’s dementia, or death with fewer years lived
with Alzheimer’s dementia.
393
Looking to the Future
Continued Population Aging
In 2011, the largest ever demographic generation of
the American population — the baby-boom generation
— started reaching age 65. By 2030, the segment of
the U.S. population age 65 and older will have grown
substantially, and the projected 71 million older
Americans will make up over 20% of the total population
(up from 17% in 2022).
237
Additionally, the size of the
older adult population is expected to continue to
increase relative to the population age 64 and younger
— a shift known as population aging — due to a
projected decline in fertility, as well as to mortality
improvements at older ages. Fertility, the average
number of children per woman, has decreased since
1960 in the United States.
394
With fewer babies born
each year, older adults will make up a larger proportion
of the population. Because increasing age is the
predominant risk factor for Alzheimer’s dementia, as the
number and proportion of older Americans grows
rapidly, so too will the numbers of new and existing cases
of Alzheimer’s dementia, as shown in Figure 5, page 32.
A5, 241
By 2060, the number of people age 65 and older with
Alzheimer’s dementia is projected to reach 13.8 million,
barring the development of medical breakthroughs to
prevent or cure Alzheimer’s disease.
A5, 241
Growth of the Age 85 and Older Population
The number of Americans in their 80s, 90s and beyond
is expected to grow dramatically due to population
aging.
237
This will lead to an increase in the number and
percentage of Americans 85 and older. This age group
is expected to comprise 11% of the population age 65
and older in 2025 and 21% of the population age 65
and older in 2050.
395
This will result in an additional
10 million people age 85 and older — individuals at the
highest risk for developing Alzheimer’s dementia.
395
• In 2024, about 2.4 million people living with Alzheimer’s
dementia are expected to be age 85 or older,
accounting for 34% of all people with Alzheimer’s
dementia.
241
• By 2060, 6.7 million people age 85 and older are
expected to have Alzheimer’s dementia, accounting
for about half (48%) of all people 65 and older with
Alzheimer’s dementia.
241
Increased Diversity of Older Adults
The group of older adults who will be at risk for
Alzheimer’s in the coming years will be socially,
culturally and economically different from previous
groups of older U.S. adults. For example, between 2018
and 2040, projections for older adults show increases
in the American Indian population of 75%, in the Black
population of 88%, in the Asian population of 113% and
in the Hispanic population of 175% compared with an
increase of 32% in the White population.
396
In addition, in the coming decades women age 65 and
older will be among the first generations of women
to have widely worked outside the home, and they will
have more years of formal education than previous
generations of women.
397
In parallel these generations
of women came of age during a decrease in the birth
rate, resulting in smaller family size.
398
Whether and
how these social and economic experiences influence
women’s risk of and resilience to Alzheimer’s will
become clearer in the decades ahead.
Since the 1970s, the gap in income in the U.S. between
lower-income, middle-income, and upper-income
households has been widening.
399
This means that the
many people who are age 65 and over experienced
their adulthood during this trend, which may have
influenced health and health behaviors prior to age 65.
In older adulthood, income inequality may influence a
wealth gap, which may have implications for health care,
health behaviors, and social determinants of health that
influence Alzheimer’s risk in particular among low-
income households.
Given the different life experiences of future older adult
populations, it is unclear what the accompanying changes
will be to dementia incidence and prevalence, both at the
population level and within racial/ethnic, socioeconomic,
and sex and gender groups. A birth cohort perspective,
which considers how a certain group of people has passed
through different stages of life in particular years, will be
increasingly important for understanding factors of risk
and resilience that may be unique to the groups of people
at risk for dementia in the coming decades.
400-402
Prevalence 33

Mortality and Morbidity
Among people age 70, 61% of
those with Alzheimer’s dementia
are expected to die before age 80
compared with 30% of people
without Alzheimer’s dementia.

Alzheimer’s disease was the fifth-leading
cause of death among individuals age 65 and
older in 2021.
403
Alzheimer’s disease may
cause even more deaths than official sources
recognize. It is also a leading cause of disability
and poor health (morbidity) in older adults.
405
Before a person with Alzheimer’s dies, they
are likely to live through years of morbidity
as the disease progresses.
Alzheimer’s disease was officially listed as the sixth-leading
cause of death in the United States in 2019;
403
in the years
2020 and 2021, when COVID-19 became the third-leading
cause of death, Alzheimer’s disease was the seventh-leading
cause of death. Official counts for more recent years are still
being compiled.
404
Deaths from Alzheimer’s Disease
The data presented in this section are through 2021, the
latest year for which finalized death data are available.
Starting in 2020, the COVID-19 pandemic had a dramatic
effect on deaths in the United States (see the box “The
Effect of the COVID-19 Pandemic on Deaths from
Alzheimer’s Disease” for a discussion of the effect of the
pandemic on Alzheimer’s mortality). In 2021, Alzheimer’s
mortality trends started to more closely resemble the
year-by-year trends from before the COVID-19 pandemic.
In this section, “deaths from Alzheimer’s disease” refers to
what is officially reported on death certificates. Note that
while death certificates use the term “Alzheimer’s disease,”
the determination is made based on clinical symptoms
in almost every case, and thus more closely aligns with
“Alzheimer’s dementia” as we have defined it in previous
sections of this report; to remain consistent with the
CDC terminology for causes of death, we use the term
“Alzheimer’s disease” for this section when referring to
officially reported statistics gleaned from death certificates.
It is difficult to determine how many deaths are caused by
Alzheimer’s disease each year because of the way causes
of death are recorded. According to data from the Centers
for Disease Control and Prevention (CDC), 119,399
people died from Alzheimer’s disease in 2021.
403
The CDC
considers a person to have died from Alzheimer’s if the
death certificate lists Alzheimer’s as the underlying cause
of death, defined as “the disease or injury which initiated
the train of events leading directly to death.”
406
The number of deaths from dementia of any type is
much higher than the number of reported Alzheimer’s
deaths. In 2021, some form of dementia was the
officially recorded underlying cause of death for 279,704
individuals (this includes the 119,399 from Alzheimer’s
disease).
403
Therefore, the number of deaths from all
causes of dementia, even as listed on death certificates,
is more than twice as high as the number of reported
Alzheimer’s deaths alone.
Severe dementia frequently causes complications such
as immobility, swallowing disorders and malnutrition that
significantly increase the risk of acute conditions that can
cause death. One such condition is pneumonia (infection
of the lungs), which is the most commonly identified
immediate cause of death among older adults with
Alzheimer’s or other dementias.
407-410
One pre-COVID-19
autopsy study found that respiratory system diseases
were the immediate cause of death in more than half of
people with Alzheimer’s dementia, followed by circulatory
system disease in about a quarter.
408
Death certificates
for individuals with Alzheimer’s often list acute conditions
such as pneumonia as the primary cause of death rather
than Alzheimer’s.
408, 409
As a result, people with Alzheimer’s
dementia who die due to these acute conditions may not
be counted among the number of people who die from
Alzheimer’s disease, even though Alzheimer’s disease may
well have caused the acute condition listed on the death
certificate. This difficulty in using death certificates to
determine the number of deaths from Alzheimer’s and other
dementias has been referred to as a “blurred distinction
between death with dementia and death from dementia.”
411
Another way to determine the number of deaths from
Alzheimer’s dementia is through calculations that
compare the estimated risk of death in those who
have Alzheimer’s dementia with the estimated risk of
death in those who do not have Alzheimer’s dementia.
A study using data from the Rush Memory and Aging
Project and the Religious Orders Study estimated that
500,000 deaths among people age 75 and older in the
United States in 2010 could be attributed to Alzheimer’s
dementia (estimates for people age 65 to 74 were not
available), meaning that those deaths would not be
Mortality and Morbidity 35

36 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
The Effect of the COVID-19 Pandemic on Deaths from Alzheimer’s Disease
In 2020 and 2021, COVID-19 was the third-leading cause of
death in the United States, pushing Alzheimer’s disease from
the sixth- to the seventh-leading cause of death.
404
Data for
more recent years were still being compiled as of the time this
report was written. Despite the change in rankings on the list
of causes of death, the total number of deaths from Alzheimer’s
disease recorded on death certificates increased 10.5% between
2019 and 2020 to 134,242.
403
COVID-19 was likely a significant
contributor to the large increase in deaths from Alzheimer’s.
Data from the Centers for Disease Control and Prevention
(CDC) show that excess mortality (the difference between the
observed number of deaths and the expected number of deaths
during a given period) from any cause has been very high during
the height of the pandemic, especially among older adults.
416
Many of these excess deaths were in vulnerable older adults
with Alzheimer’s disease and other dementias. Among Medicare
beneficiaries age 65 and over with Alzheimer’s disease and other
dementias, overall mortality increased 26% between 2019 and
2020, which is twice as high as the increase among beneficiaries
without Alzheimer’s disease and other dementias.
417
Furthermore,
increased mortality between 2019 and 2020 among Medicare
beneficiaries with Alzheimer’s disease and related dementia
was greater among Black, Hispanic and Asian beneficiaries than
among White beneficiaries and the nursing home population.
417
As shown in Figure 6, compared with the average annual number
of deaths in the five years before 2020, there were 13,925 more
deaths from Alzheimer’s disease and 44,729 more deaths from
all dementias, including Alzheimer’s, in 2020. This is, respectively,
12% and 17% more than expected.
403
In 2021, there were 1,082
more deaths from Alzheimer’s disease and 20,449 more deaths
from all dementias compared with the average of the five years
before 2020.
403
The number of people dying from Alzheimer’s
has been increasing over the last two decades, but the number
of excess deaths from Alzheimer’s disease in 2020 far exceeded
what would have been expected from this pre-pandemic trend.
The number for 2021, by contrast, is closer to the pre-pandemic
trend. Data for more recent years are still being compiled, but one
study found that deaths due to all dementias, including Alzheimer’s,
decreased between March 2021 and February 2022, in particular
among residents of nursing homes and long-term care facilities.
418
The impact of COVID-19 can also be seen when examining the
number of deaths from COVID-19 for which death certificates
also listed Alzheimer’s or another dementia as a cause of death
(referred to as a “multiple cause of death”). In 2020 and 2021,
1 in every 10 death certificates listing COVID-19 as the primary
cause of death also listed Alzheimer’s disease or another dementia
as a multiple cause of death. Among people age 85 or older who
died of COVID-19 in 2020 or 2021, Alzheimer’s disease or
another dementia was listed as a multiple cause of death on
almost a quarter of death certificates.
404
The COVID-19 pandemic had a dramatic effect on mortality
from Alzheimer’s and other dementias. Nursing homes and other
long-term care facilities were the site of major outbreaks in the
early stages of the pandemic, and residents with Alzheimer’s
and other dementias were particularly vulnerable. What remains
unclear is whether and how this will affect the longer-term trend
in deaths from Alzheimer’s now that the COVID-19 pandemic has
subsided. As the pandemic has progressed and COVID-19 is no
longer as fatal for most people, the question of “dying with” or
“dying from” COVID-19 is getting harder to parse. In many ways
this echoes the discussion about dying with or from Alzheimer’s
disease discussed in this section. What is clear is that for at least
the first years of the pandemic, having Alzheimer’s or another
dementia made older adults more vulnerable to COVID-19 and
increased the risk of dying from COVID-19.
2015-2019 Average 2020 2021
Deaths
*Data for 2021 are as of February 7, 2022.
Created from data from the National Center for Health Statistics.
416
8,000
6,000
4,000
2,000
0
Month
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov
Dec
Deaths Due to Alzheimer’s and Other Dementias in the United States in 2020 and 2021 Compared with Previous Years*
Figure
6

37
expected to occur in that year if the individuals did not
have Alzheimer’s dementia.
407
A more recent study using
data from the nationally representative Health and
Retirement Study (HRS) estimated that about 14% of
deaths among Americans age 70 and older from 2000-
2009 were attributable to dementia, while only 5% of
death certificates listed dementia as the underlying cause
of death for this age group, indicating underreporting
on death certificates.
412
According to 2019 Medicare
claims data, about one-third of all Medicare beneficiaries
who die in a given year have been diagnosed with
Alzheimer’s or another dementia.
413
Based on data from
the Chicago Health and Aging Project (CHAP) study, in
2020 an estimated 700,000 people age 65 and older in
the United States had Alzheimer’s dementia at death.
414
Although some undoubtedly died from causes other than
Alzheimer’s, it is likely that many died from Alzheimer’s
disease itself or from conditions for which Alzheimer’s
was a contributing cause, such as pneumonia. Thus,
taken together, the specific number of deaths caused by
Alzheimer’s is unknown.
Adding further complexity, the vast majority of death
certificates listing Alzheimer’s disease as an underlying
cause of death are not verified by autopsy, and research
has shown that 15% to 30% of those diagnosed with
Alzheimer’s dementia during life do not have the brain
changes of Alzheimer's disease but instead have the
Mortality and Morbidity
Created from data from the National Center for Health Statistics.
403,419
1.2%
Percentage
160
140
120
100
80
60
40
20
0
-20
-40
-60
-80
Breast
cancer
Cause
of death
Prostate
cancer
Heart
disease
Stroke HIV Alzheimer’s
disease
140.9%
-65.6%
-2.8%
-2.1%
4.8%
Percentage Changes in Selected Causes of Death (All Ages) Between 2000 and 2021
This report keeps the racial, ethnic and other
population identifiers used in source documents
when describing findings from specific studies.
Figure
7

38 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Number of Deaths and Annual Mortality Rate (per 100,000 People) Due to Alzheimer’s Disease by State, 2021
Created from data from the National Center for Health Statistics.
A6,403
State
Number
of Deaths
Mortality
Rate
Alabama 2,725 54.1
Alaska 135 18.4
Arizona 2,754 37.8
Arkansas 1,559 51.5
California 16,911 43.1
Colorado 1,778 30.6
Connecticut 1,049 29.1
Delaware 381 38.0
District of Columbia 66 9.9
Florida 6,716 30.8
Georgia 4,378 40.5
Hawaii 562 39.0
Idaho 789 41.5
Illinois 4,025 31.8
Indiana 2,238 32.9
Iowa 1,185 37.1
Kansas 805 27.4
Kentucky 1,632 36.2
Louisiana 2,121 45.9
Maine 539 39.3
Maryland 1,129 18.3
Massachusetts 1,558 22.3
Michigan 4,198 41.8
Minnesota 2,251 39.4
Mississippi 1,694 57.4
Missouri 2,517 40.8
State
Number
of Deaths
Mortality
Rate
Montana 341 30.9
Nebraska 687 35.0
Nevada 804 25.6
New Hampshire 422 30.4
New Jersey 2,399 25.9
New Mexico 634 30.0
New York 3,582 18.1
North Carolina 4,260 40.4
North Dakota 325 41.9
Ohio 4,947 42.0
Oklahoma 1,580 39.6
Oregon 2,047 48.2
Pennsylvania 4,109 31.7
Rhode Island 445 40.6
South Carolina 2,419 46.6
South Dakota 396 44.2
Tennessee 2,879 41.3
Texas 10,437 35.3
Utah 998 29.9
Vermont 337 52.2
Virginia 2,582 29.9
Washington 3,644 47.1
West Virginia 851 47.7
Wisconsin 2,371 40.2
Wyoming 208 35.9
Total 119,399 36.0
Table
5

39
brain changes of another cause of dementia (see Table 1,
pages 6-7).
21, 79, 243-245
Therefore, an underlying cause of
death listed as Alzheimer’s disease may not be accurate.
Irrespective of the cause of death, among people age 70,
61% of those with Alzheimer’s dementia are expected to
die before age 80 compared with 30% of people without
Alzheimer’s dementia.
415
Public Health Impact of Deaths from
Alzheimer’s Disease
In the past two decades, although the number of
deaths from other major causes decreased significantly
or remained approximately the same, official records
indicate that deaths from Alzheimer’s disease increased
significantly. Between 2000 and 2021, the number of
deaths from Alzheimer’s disease as recorded on death
certificates more than doubled, increasing 141%, while
deaths from the number-one cause of death (heart
disease) decreased 2.1% (Figure 7).
403, 419
The increase
in the number of death certificates listing Alzheimer’s
as the underlying cause of death probably reflects two
trends: first, Alzheimer’s has become a more common
cause of death as the population ages; and second, over
time, physicians, coroners and others who assign causes
of death may be increasingly likely to report Alzheimer’s
on death certificates.
420
State-by-State Deaths from Alzheimer’s
Table 5 provides information on the number of deaths
due to Alzheimer’s by state in 2021, the most recent
year for which state-by-state data are available. This
information was obtained from death certificates and
reflects the condition identified by the physician or other
medical personnel who filled out the death certificate as
the underlying cause of death. The table also provides
annual mortality rates by state, computed with the death
certificate data, to compare the risk of death due to
Alzheimer’s disease across states with varying population
sizes. For the United States as a whole, in 2021, the
mortality rate for Alzheimer’s disease was 36 deaths per
100,000 people.
A6, 403
Alzheimer’s Death Rates
As shown in Figure 8, the annual rate of deaths due to
Alzheimer’s — that is, the number of Alzheimer’s deaths
per 100,000 people in the population — has risen
substantially since 2000.
403
Table 6 shows that the
annual rate of death from Alzheimer’s increases
dramatically with age, especially after age 65.
A6, 403
The
increase in the Alzheimer’s death rate over time has
disproportionately affected people age 85 and older.
419
Between 2000 and 2021, the death rate from
Alzheimer’s increased 41% for people age 65 to 74,
54% for people age 75 to 84 and 86% for people age 85
Mortality and Morbidity
Created from data from the National Center for Health Statistics.
403
U.S. Annual Alzheimer’s Death Rate (per 100,000 People) by Year
40
35
30
25
20
15
10
5
0
Rate
17.6
20.5
22.5
24.3
27.1
27.0
26.6
29.3
40.7
36.0
37.3
35.9
2000 2002 2004 2006 2008 2010 2012 2014 2018 2020 20212016Year
Figure
8

and older.
403
A report by the CDC determined that even
after adjusting for changes over time in the specific ages
of people within these age groups, the annual Alzheimer’s
death rate in the U.S. increased substantially between
1999 and 2014.
420
Therefore, the advancing average age
of the older adult population in the U.S. is not the only
explanation for the increase in Alzheimer’s death rates.
Other possible reasons include fewer deaths from other
common causes of death in old age such as heart disease
and stroke; increased clinical recognition of and formal
diagnosis of Alzheimer’s dementia; and increased
reporting of Alzheimer’s as a cause of death by physicians
and others who complete death certificates.
420
Duration of Illness from Diagnosis to Death
and Time Spent in Nursing Home
Studies indicate that people age 65 and older survive
an average of four to eight years after a diagnosis of
Alzheimer’s dementia, yet some live as long as 20 years
with Alzheimer’s dementia.
10-18
This reflects the slow,
insidious and uncertain progression of Alzheimer’s. A
person who lives from age 70 to age 80 with Alzheimer’s
dementia will spend an average of 40% of this time in the
severe stage.
415
Much of this time will be spent in a nursing
home (see Use and Costs section, page 70). At age 80,
approximately 75% of people with Alzheimer’s dementia
live in a nursing home compared with only 4% of the
general population age 80.
415
In all, an estimated
two-thirds of those who die of dementia do so in
nursing homes, compared with 20% of people with cancer
and 28% of people dying from all other conditions.
421
The Burden of Alzheimer’s Disease
The long duration of illness before death contributes
significantly to the public health impact of Alzheimer’s
disease because much of that time is spent in a state of
severe disability and dependence. Scientists have developed
measures that compare the burden of different diseases on
a population in a way that takes into account not only the
number of people with the condition, but also the number
of years of life lost due to that disease and the number of
healthy years of life lost by virtue of being in a state
of disability. One measure of disease burden is called
disability-adjusted life years (DALYs), which is the sum of
the number of years of life lost (YLLs) due to premature
mortality and the number of years lived with disability
(YLDs), totaled across all those with the disease or injury.
These measures indicate that Alzheimer’s is a very
burdensome disease, not only to the individuals with the
disease, but also to their families, informal caregivers and
communities at large. In recent years, the burden of
Alzheimer’s has increased more dramatically in the United
States than the burden of other diseases. According to the
most recent Global Burden of Disease classification system,
Alzheimer’s disease rose from the 12th most burdensome
disease or injury in the United States in 1990 to the sixth
in 2016 in terms of DALYs.
405
In 2016, Alzheimer’s disease
was the fourth highest disease or injury in terms of YLLs
and the 19th in terms of YLDs.
405
These estimates should be interpreted with consideration
of the comparability of data across time and
422
and how
disability is incorporated. These Alzheimer’s burden
estimates use different sources for each state in a given
Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).40
Created from data from the National Center for Health Statistics.
403
U.S. Annual Alzheimer’s Death Rates (per 100,000 People) by Age and Year
Age 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 2020 2021
45-54 0.2 0.1 0.2 0.2 0.2 0.3 0.2 0.2 0.2 0.3 0.2 0.3
55-64 2.0 1.9 1.8 2.1 2.2 2.1 2.2 2.1 2.7 2.9 3.3 3.2
65-74 18.7 19.6 19.5 19.9 21.1 19.8 17.9 19.6 23.6 24.7 28.6 26.4
75-84 139.6 157.7 168.5 175.0 192.5 184.5 175.4 185.6 214.1 213.9 229.3 214.3
85+ 667.7 790.9 875.3 923.4 1,002.2 987.1 936.1 1,006.8 1,216.9 1,225.3 1,287.3 1,243.6
Table
6
year, and data sources for states may differ over the years.
Estimates do not account for the context in which
disability is experienced, including social support and
economic resources,
423
which vary widely. Estimates may
not fully account for variation in disability levels between
individuals and along the stages of Alzheimer’s dementia.
These variations in data sources and consideration of
disability may limit the value of these metrics and the
comparability of estimates across states and across years.
Looking to the Future
Taken together, these statistics indicate that not only is
Alzheimer’s disease responsible for the deaths of more and
more Americans, but also that Alzheimer’s and other
dementias are contributing to more and more cases of poor
health and disability in the U.S. With population aging, the
percentage of deaths related to Alzheimer’s and other
dementias will likely continue to increase. The health and
well-being of people with Alzheimer’s and other dementias
should continue to be prioritized. Thus, it will remain
important to develop a comprehensive understanding of
how Alzheimer’s and other dementias contribute to poor
health, disability and mortality. That understanding requires
innovation in research methods that are more inclusive and
that fully capture the lived experience of disability of people
living with dementia and of their families and caregivers.
Mortality and Morbidity 41

Caregiving
More than million Americans
provide unpaid care for a family
member or friend with dementia,
a contribution to the nation
valued at nearly $ billion
.

43
When supporting a person living with
Alzheimer’s dementia, caregiving often includes
assistance with one or more activities of daily
living (ADLs), such as bathing and dressing, as
well as multiple instrumental activities of daily
living (IADLs), such as paying bills, shopping and
using transportation.
424, 425
Caregivers also
provide emotional support to people with
Alzheimer’s dementia, help them manage health
conditions, and communicate and coordinate
care with other family members and health care
providers (see Table 7). In addition to providing
descriptive information about caregivers of
people with Alzheimer’s or other dementias,
this section characterizes caregivers of people
with dementia in comparison with either
caregivers of people with other medical
conditions or, if that comparison is not available,
with people who are not caregivers (referred to
here as non-caregivers).
Unpaid Caregivers
Eighty-three percent of the help provided to older adults
in the United States comes from family members, friends
or other unpaid caregivers.
426
Nearly half of all caregivers
(48%) who provide help to older adults do so for someone
with Alzheimer’s or another dementia.
427
More than
11 million Americans provide unpaid care for people with
Alzheimer's or other dementias.
A7
Table 8 provides details
about unpaid caregivers.
In 2023, caregivers of people with Alzheimer’s or other
dementias provided an estimated 18.4 billion hours
A8
of
informal — that is, unpaid — assistance, a contribution
valued at $346.6 billion.
A9
This is approximately 57% of the
net value of Walmart’s total revenue in fiscal year 2023
($611.3 billion)
428
and nearly 15 times the total revenue of
McDonald’s in 2022 ($23.3 billion).
429
The total lifetime cost
of care for someone with dementia was estimated at almost
$400,000 in 2023 dollars. Seventy percent of this lifetime
cost of care is borne by family caregivers in the forms of
unpaid caregiving and out-of-pocket expenses for items
ranging from medications to food for the person with
Caregiving refers to attending to another
person’s health needs and well-being.
dementia. Remaining costs encompass payments by
Medicare and Medicaid (see the Use and Costs of Health Care,
Long-Term Care and Hospice section, page 70).
430, 431
Current
estimates of the lifetime costs of care may underestimate
the financial impact of a relative’s dementia on family
caregivers’ health and workplace productivity, as other
potential costs such as home modifications, respite service
use, and health/work productivity challenges are not always
considered in cost estimates.
432
Caregiving
Helping with instrumental activities of daily living
(IADLs), such as household chores, shopping, preparing
meals, providing transportation, arranging for doctor’s
appointments, managing finances and legal affairs, and
answering the telephone.
Helping the person take medications correctly, either via
reminders or direct administration of medications.
Helping the person adhere to treatment recommendations
for dementia or other medical conditions.
Assisting with personal activities of daily living (ADLs), such
as bathing, dressing, grooming and feeding and helping the
person walk, transfer from bed to chair, use the toilet and
manage incontinence.
Managing behavioral symptoms of the disease such as
aggressive behavior, wandering, depressive mood, agitation,
anxiety, repetitive activity and nighttime disturbances.
Finding and using support services such as support groups
and adult day service programs.
Making arrangements for paid in-home, nursing home or
assisted living care.
Hiring and supervising others who provide care.
Assuming additional responsibilities that are not necessarily
specific tasks, such as:
• Providing overall management of getting through the day.
• Addressing family issues related to caring for a relative
with Alzheimer’s disease, including communication with
other family members about care plans, decision-making
and arrangements for respite for the main caregiver.
• Managing other health conditions (i.e., “comorbidities”),
such as arthritis, diabetes or cancer.
• Providing emotional support and a sense of security.
Dementia Caregiving Tasks
Table
7

44 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Among the reasons shared by caregivers for providing
assistance to a person with Alzheimer’s or another
dementia are the desire to keep a family member or friend
at home (65%), close proximity to the person with dementia
(48%), and the caregiver’s perceived obligation to the
person with dementia (38%).
A10
In addition, caregivers often
indicate love and a sense of duty when describing what
motivates them to assume care responsibilities for a
relative or friend living with dementia.
433
Individuals with dementia living in the community are
more likely than older adults without dementia to rely on
multiple unpaid caregivers (often family members); 30%
of older adults with dementia rely on three or more unpaid
caregivers, whereas 23% of older adults without dementia
do so.
434
Only a small percentage (8%) of older adults with
dementia do not receive help from family members or
other informal care providers. Of these individuals,
nearly half live alone, perhaps making it more difficult to
ask for and receive informal care.
434
Among caregivers
of spouses with dementia who are at the end of life,
close to half provide care without the help of other family
or friends.
435
Living alone with dementia may be a particular challenge
for certain subgroups, such as lesbian, gay, bisexual
and transgender (LGBT) individuals, who may experience
greater isolation due to potential social stigma and
a diminished social network of available family or
friend caregivers.
436-439
Caregiving and Women
The responsibilities of caring for someone with dementia
often fall to women. Approximately two-thirds of dementia
caregivers are women.
A10, 440, 441, 446
Findings from the 2018
National Health and Wellness survey indicated that more
dementia caregivers in the United States are women
(61.5%) than in Japan (51.9%) or five European countries/
regions (56.3%: France, Germany, the United Kingdom, Italy
and Spain).
448
Over one-third of dementia caregivers in the
United States are daughters caring for a parent.
426, 434
It is
more common for wives to provide informal care for a
husband than vice versa.
449
On average, female caregivers
spend more time caregiving than male caregivers.
434
The
2015-2017 BRFSS surveys found that of all dementia
caregivers who spend more than 40 hours per week
providing care, 73% were women.
443
Two and a half times
as many women as men reported living with the person
with dementia full time.
450
Of those providing care to
someone with dementia for more than five years, 63%
were women.
443
Similarly, caregivers who are women may
experience slightly higher levels of burden, impaired mood,
depression and impaired health than do caregivers who
are men, with evidence suggesting that these differences
arise because female caregivers tend to spend more time
caregiving, assume more caregiving tasks, and care for
someone with more cognitive, functional and/or behavioral
problems.
451-453
Among dementia caregivers who indicated
a need for individual counseling or respite care, the large
majority were women (individual counseling, 85%, and
respite care, 84%).
443
Sex/gender
•
Approximately two-thirds of dementia caregivers are women.
A10, 440, 441
Race/ethnicity
•
Two-thirds of caregivers are White,
A10, 441, 442
10% are Black, 8% are Hispanic, and 5% are
Asian American.
A10
The remaining 10% represent a variety of other racial/ethnic groups.
Living status
•
Most caregivers (66%) live with the person with dementia in the community.
434
•
Over 60% of caregivers are married, living with a partner or in a long-term relationship.
A10, 441
•
Approximately one-quarter of dementia caregivers are “sandwich generation” caregivers — meaning that
they care not only for an aging parent but also for at least one child.
A10, 442, 443
Caring for parents
•
Over half of caregivers are providing assistance to a parent or in-law with dementia.
442
•
Among primary caregivers (individuals who indicate having the most responsibility for helping their relatives)
of people with dementia, over half take care of their parents.
444-446
Income
•
Forty-one percent of caregivers have a household income of $50,000 or less.
A10
Education
•
Approximately 40% of dementia caregivers have a college degree or more of education.
A10, 441, 442
Age
•
About 30% of caregivers are age 65 or older.
A10
•
Twenty-three percent of caregivers ages 18 to 49 help someone with dementia, which is an increase of
7% between 2015 and 2021.
447
Caring for spouse
•
Approximately 10% of caregivers provide help to a spouse with Alzheimer’s disease or another dementia.
442
Who Are the Caregivers?
Table
8

45
Race, Ethnicity and Dementia Caregiving
A1
Only recently have population-based studies examined
racial disparities in dementia caregiving. Close to half of
Black and Hispanic individuals with dementia live with adult
children (47.1%), compared with less than a quarter of
White individuals with dementia (24.6%).
454
Compared
with White caregivers, Black caregivers are more likely
to provide more than 40 hours of care per week
(54.3% versus 38.6%) and care for someone with dementia
(31.7% versus 11.9%). Black dementia caregivers are
also more likely to provide help with ADLs than White
dementia, White non-dementia, and Black non-dementia
caregivers.
455, 456
Black male dementia caregivers are
3.3 times more likely to experience financial burdens when
compared with Black female and White male and female
dementia caregivers, whereas Black and White male
dementia caregivers are 37% to 71% less likely than White
female dementia caregivers to indicate emotional
burden.
457
Black dementia caregivers were found to be 69%
less likely than White caregivers to use respite services,
although the need for dementia care relief is considerable
among Black families.
458, 459
Hispanic, Black and Asian
American dementia caregivers indicate greater care
demands, less outside help/formal service use and greater
depression compared with White caregivers.
460-462
In a
nationally representative study,
463
Black and Hispanic
participants had poorer health prior to becoming a
caregiver for a spouse with dementia than those of similar
race/background who did not become caregivers; such
differences were not apparent among White caregivers.
Discrimination is also linked with depressive symptoms
among African American dementia caregivers.
464
Black caregivers are more likely than White caregivers to
report positive aspects of caregiving.
455
A meta-analysis
found that Black dementia caregivers indicate slightly
higher psychological well-being than White dementia
caregivers. Hispanic dementia caregivers, however,
reported slightly lower physical well-being than White
dementia caregivers.
465
Other research has examined
variations in self-rated health among dementia caregivers
of diverse racial and ethnic backgrounds. Support from
family and friends is associated with better self-rated
health for Black dementia caregivers but not for White or
Hispanic caregivers.
460
Having a more positive perceived
relationship between the caregiver and person with
dementia was associated with better self-rated health
among Black and White caregivers.
460
Non-Hispanic Black
dementia family caregivers are less likely to exercise and
live with diabetes than non-Hispanic White and non-
Hispanic Asian dementia family caregivers.
466
The need for culturally informed theories, research
frameworks, and services for people living with dementia
and their caregivers is pronounced.
467-471
Cultural values
(e.g., familismo: the Latino cultural value of placing family
needs and loyalty to one’s family above one's own needs)
may influence disparities in perceptions and use of support
among caregivers across diverse racial and ethnic
contexts.
472, 473
Underutilization of needed services on the
part of Latino dementia caregivers may be due to culturally
incongruent expectations on the part of health care
systems and providers that assume that families are the
predominant/only support network for Latino individuals
with dementia.
474
Black/African-American dementia
caregivers' needs include greater education about
dementia treatment, diagnosis, and care strategies;
navigating what is often perceived as a “broken” health care
system; improved access to affordable transportation and
health care services; greater education about navigation of
family conflict; increased availability of respite support;
better communication about dementia within the Black/
African-American community; and increased availability of
financial/legal planning.
459, 475-477
Dementia caregiving is experienced by many, regardless of
race or ethnicity. The comparisons above suggest that the
experience of caregiving often varies depending on racial
and ethnic context. Studies of caregivers often lack
sufficient numbers of diverse participants to confirm these
findings or delve deeper into them for important insights.
Recent reviews and national summits have emphasized the
need to revise recruitment strategies to capture the range
of dementia care experiences among caregivers of diverse
racial and ethnic identity.
462
If representation in dementia
care research is not improved, our ability to generalize
findings or determine whether findings vary by diverse
subgroups is not possible. This hinders the progress of all
dementia caregiving research. Furthermore, if individuals
continue to lack representation in dementia research, they
will not receive the benefits of racially and ethnically
sensitive prevention, treatment or care innovations.
460, 462
Establishing stronger relationships with existing organizations
and resources in Black communities, indigenous communities
and other communities of color offers the potential for
research-based partnerships to enhance representation in
dementia research and result in more culturally appropriate
and effective services.
468, 474, 478-490
Caregiving Tasks
The care provided to people with Alzheimer’s or other
dementias is wide-ranging and in some instances all-
encompassing. Table 7, page 43, summarizes some of the
most common types of dementia care provided.
Caregiving
This report keeps the racial, ethnic and other
population identifiers used in source documents
when describing findings from specific studies.

46 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Created from data from the National Alliance for Caregiving in Partnership with the Alzheimer’s Association.
442
Although the care provided by family members of people
with Alzheimer’s or other dementias can be similar to that
provided by caregivers of people with other conditions,
dementia caregivers tend to provide more extensive
assistance.
491
Family caregivers of people with dementia are
more likely to monitor the health of the care recipient than
are caregivers of people without dementia (79% versus
66%).
492
Data from the 2011 National Health and Aging
Trends Study indicated that caregivers of people with
dementia are more likely than caregivers of people without
dementia to provide help with self-care and mobility
(85% versus 71%) and health or medical care (63% versus
52%).
427, 440
Figure 9 illustrates how caregivers of people
with dementia are more likely than caregivers of other
older people to assist with ADLs.
442
People with dementia tend to have larger networks of
family and friends involved in their care compared with
people without dementia. More family members and
friends in dementia care networks tend to provide help
for household activities, mobility and functional needs,
and transportation than family members and friends in
non-dementia care networks.
493
When a person with Alzheimer’s or another dementia
moves to an assisted living residence or a nursing home,
the help provided by his or her family caregivers usually
changes from the comprehensive care summarized in
Table 7 to providing emotional support, interacting with
residential care staff and advocating for appropriate care.
However, some family caregivers continue to help with
bathing, dressing and other ADLs.
494, 495
Duration of Caregiving
One national poll found that 86% of dementia caregivers
provided assistance for at least the past year.
A10
According
to another study, well over half (57%) of family caregivers
of people with Alzheimer’s or other dementias living in the
community had provided care for four or more years.
434
Hours of Unpaid Care and Economic Value of Caregiving
In 2023, the 11.5 million family and other unpaid
caregivers of people with Alzheimer’s or other dementias
provided an estimated 18.4 billion hours of unpaid help.
This number represents an average of nearly 31 hours of
care per caregiver per week, or 1,612 hours of care per
caregiver per year.
A8
With this care valued at the average
of the state minimum wage and the median hourly cost of
a home health aide (a conservative estimate),
A9
the
estimated economic value of care provided by family and
other unpaid caregivers of people with dementia across the
United States was $346.6 billion in 2023. Table 9, page 47,
shows the total hours of unpaid care as well as the value of
care provided by family and other unpaid caregivers for the
50
40
30
20
10
0
Getting in and out
of beds and chairs
Dealing with
incontinence
Activity Bathing or
showering
Feeding Getting to and
from the toilet
Getting
dressed
Caregivers of people with Alzheimer’s or other dementias
Percentage Caregivers of other older people
45%
43%
38%
30%
34%
23%
33%
20%
32%
25%
32%
12%
Proportion of Caregivers of People with Alzheimer’s or Other Dementias Versus Caregivers of
Other Older People Who Provide Help with Specific Activities of Daily Living, United States, 2015
Figure
9

47Caregiving
*State totals do not add to the U.S. totals due to rounding.
Created from data from the 2016, 2020, 2021, and 2022 Behavioral Risk Factor Surveillance System survey,
U.S. Census Bureau, National Alliance for Caregiving, AARP, U.S. Department of Labor and Genworth.
A7,A8,A9
State
Number of
Caregivers
(in thousands)
Hours of
Unpaid Care
(in millions)
Value of
Unpaid Care
(in millions
of dollars)
Alabama 217 387 $5,310
Alaska 25 39 796
Arizona 292 483 10,228
Arkansas 155 270 4,448
California 1,373 1,864 44,272
Colorado 177 307 7,249
Connecticut 128 201 4,331
Delaware 31 46 909
District of Columbia 14 15 343
Florida 840 1,321 24,437
Georgia 374 755 11,417
Hawaii 60 91 1,907
Idaho 66 105 1,875
Illinois 311 480 9,840
Indiana 216 322 5,186
Iowa 98 125 2,284
Kansas 89 125 1,989
Kentucky 157 302 4,869
Louisiana 168 256 3,428
Maine 51 87 1,911
Maryland 247 405 8,144
Massachusetts 213 246 5,668
Michigan 380 872 17,044
Minnesota 164 225 5,276
Mississippi 93 175 2,380
Missouri 223 350 6,478
State
Number of
Caregivers
(in thousands)
Hours of
Unpaid Care
(in millions)
Value of
Unpaid Care
(in millions
of dollars)
Montana 17 25 $478
Nebraska 40 62 1,188
Nevada 84 142 2,681
New Hampshire 48 77 1,529
New Jersey 272 494 10,882
New Mexico 67 118 2,142
New York 543 879 18,996
North Carolina 373 723 10,939
North Dakota 19 25 465
Ohio 414 624 11,427
Oklahoma 108 189 3,099
Oregon 170 229 5,285
Pennsylvania 465 822 13,668
Rhode Island 36 51 1,132
South Carolina 219 361 5,550
South Dakota 27 34 716
Tennessee 369 499 7,804
Texas 1,016 1,532 23,937
Utah 112 132 2,465
Vermont 19 28 615
Virginia 342 662 12,572
Washington 247 378 9,499
West Virginia 65 115 1,585
Wisconsin 205 297 5,528
Wyoming 16 21 385
U.S. Total 11,457 18,376 $346,585
Number of Caregivers of People with Alzheimer’s or Other Dementias, Hours of Unpaid Care
and Economic Value of Unpaid Care by State, 2023*
Table
9

48 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
United States and each state. Unpaid caregivers of people
with Alzheimer’s or other dementias provided care valued
at more than $5 billion in each of 25 states. Unpaid
caregivers provided care valued at more than $18 billion in
each of the four most populous states — California, Texas,
Florida and New York. A longitudinal study of the monetary
value of family caregiving for people with dementia found
that the overall value of daily family care increased 18% with
each additional year of providing care, and that the value of
this care further increased as the care recipient’s cognitive
abilities declined.
496
In contrast, family care costs are
reduced up to 24% in situations where caregivers
indicated they were emotionally close to the person with
dementia.
497
More research is needed to estimate the
future value of family care for people with Alzheimer’s
disease and other dementias as the U.S. population
continues to age, particularly since many existing
economic studies only consider primary caregivers when
there are often multiple relatives and others helping an
older person with dementia.
434, 498
Apart from its long duration, caregiving involves demands
that are time-intensive. Caregivers of people with dementia
report providing 27 hours more care per month on
average (92 hours versus 65 hours) than caregivers of
people without dementia.
440
An analysis of national
caregiving trends from 1999 to 2015 found that among
dementia caregivers the average hours of care per week
increased from 45 in 1999 to 48 in 2015; among non-
dementia caregivers, weekly hours of care decreased from
34 to 24.
499
The amount of time required for caregiving
increases as dementia progresses; one study showed that
people with dementia required 151 hours of caregiving
per month at the outset of dementia and this increased to
283 hours per month eight years later. This is an increase
from approximately 5 hours a day to 9 hours a day (it is
important to note that some family members/caregivers
provide assistance to someone due to cognitive issues
before a formal diagnosis of Alzheimer’s disease or a
related dementia).
500, 501
Each instance of a decrease in
ADL or IADL function in someone with dementia results
in nearly five more hours of monthly caregiving compared
with a similar functional decrease for someone without
dementia.
502
Over a two-year period, one national study
found that impairment in one additional self-care activity
(e.g., bathing, dressing, eating and using the toilet) for
those with dementia resulted in 28 additional hours
of family care required per month; for those without
dementia, an additional self-care need was associated
with an increase in 15 hours of family care per month.
503
Health and Economic Impacts of Alzheimer’s Caregiving
Caring for a person with Alzheimer’s or another dementia
poses special challenges. For example, people in the
moderate to severe stages of Alzheimer’s dementia
experience losses in judgment, orientation, and the ability
to understand and communicate effectively. Family
caregivers must often help people with dementia manage
these issues. The personality and behavior of a person with
dementia are affected as well, and these changes are often
among the most challenging for family caregivers.
504-506
Individuals with dementia also require increasing levels of
supervision and personal care as the disease progresses. As
the person with dementia’s symptoms worsen, caregivers
can experience increased emotional stress and depression;
new or exacerbated health problems; and depleted income
and finances due in part to disruptions in employment and
paying for health care or other services for both
themselves and the person living with dementia.
507-514
Caregiver Emotional and Social Well-Being
The intimacy, shared experiences and memories that
are often part of the relationship between a caregiver
and person living with dementia may be threatened due
to the memory loss, functional impairment and
psychiatric/behavioral disturbances that can accompany
the progression of dementia. In the 2017 National Poll
on Healthy Aging, however, 45% of caregivers of people
with dementia indicated that providing help to someone
with cognitive impairment was very rewarding.
446
In the
2011 National Study of Caregiving, greater satisfaction
from dementia caregiving was associated with more
emotional support from family members and friends.
515
Although caregivers report positive feelings about
caregiving, such as family togetherness and the
satisfaction of helping others,
A10, 516-524
they also
frequently report higher levels of burden and stress;
depression or other adverse mental health outcomes;
strain; and problems with navigating care transitions
when compared with other caregivers or non-caregivers.
Created from data from the Alzheimer’s Association.
A10
59%
60
40
20
0
Emotional stress
of caregiving
Stress Physical stress
of caregiving
38%
Percentage
Percentage of Dementia Caregivers Who Report
High to Very High Stress Due to Caregiving
Figure
10

49
Burden and Stress
• Compared with caregivers of people without
dementia, caregivers of those with dementia indicate
more substantial emotional, financial and physical
difficulties.
440, 491
• Fifty-nine percent of family caregivers of people with
Alzheimer’s or other dementias rated the emotional
stress of caregiving as high or very high (Figure 10).
A10
• Spousal dementia caregivers are more likely than
non-spousal dementia caregivers to experience
increased burden over time. This increased burden
also occurs when the person with dementia develops
behavioral changes and decreased functional ability.
525
• Many people with dementia have co-occurring
chronic conditions, such as hypertension or arthritis,
which may complicate caregiving. For example, a
national study found that caregivers of people with
dementia who had a diagnosis of diabetes or
osteoporosis were 2.6 and 2.3 times more likely,
respectively, to report emotional difficulties with care
compared with caregivers of people with dementia
who did not have these co-occurring conditions.
526
Depression and Mental Health (see also Table 10, page 51)
• A meta-analysis reported that caregivers of
people with dementia were significantly more
likely to experience depression and anxiety than
non-caregivers.
453
Dementia caregivers also indicate
more depressive symptoms than non-dementia
caregivers.
527
• The prevalence of depression is higher among
dementia caregivers (30% to 40% as reported in
multiple studies) than other caregivers, such as those
who provide help to individuals with schizophrenia
(20%) or stroke (19%).
528, 529
• Caring for a spouse with dementia is associated with a
30% increase in depressive symptoms compared with
spousal caregivers of partners without dementia.
530
• In a meta-analysis, the type of relationship was the
strongest predictor of caregiver depression;
caregivers of spouses with dementia had two-and-
a-half times higher odds of having depression than
caregivers of people with dementia who were
not spouses.
528
• The prevalence of anxiety among dementia
caregivers is 44%, which is higher than among
caregivers of people with stroke (31%).
528
• Dementia caregivers in the United States were more
likely to have experienced depression (32.5%) or
anxiety (26%) when compared with dementia
caregivers from Japan (16.8% and 12.9%, respectively)
or those from across Germany, Italy, Spain, France
and the United Kingdom (29.3% for depression and
22.4% for anxiety).
448
• Caregivers of individuals with Alzheimer’s report
more subjective cognitive problems (for example,
problems with memory) and experience greater
declines in cognition over time than non-caregivers
matched on age and other characteristics.
531, 532
• Caring for people with dementia who have four or
more behavioral and psychological symptoms (for
example, aggression, self-harm and wandering)
represents a “tipping point,” as these caregivers are
more likely to report clinically meaningful depression
and burden.
533
• A systematic review found the prevalence of suicidal
ideation (thinking about or making plans for suicide)
in dementia caregivers with a mean age of 64 was
32% compared with 2.7% in U.S. adults age 56 and
older (please note that an exact age comparator is
not available).
534, 535
• Providing physical and medical care is associated with
worse mental health among dementia caregivers than
non-dementia caregivers.
491
Other Key Findings About the Challenges of
Dementia Caregiving
• Caregivers of people with Alzheimer’s or other
dementias are twice as likely as caregivers of
individuals without dementia (22% compared with
11%) to report that completing medical/nursing-
related tasks (for example, injections, tube feedings
and catheter/colostomy care) was difficult.
492
• Dementia caregivers often experience challenges
managing medications for individuals with dementia,
such as non-adherence.
536-539
• Compared with non-dementia caregivers, dementia
caregivers indicate a greater decrease in their social
networks (e.g., other relatives, friends, acquaintances).
540
• According to a national Alzheimer’s Association poll
of caregivers, respondents often believed they had
no choice in taking on the role of caregiver.
A10
• The poll also found that more than half (53%) of
women with children under age 18 felt that
caregiving for someone with dementia was more
challenging than caring for children.
A10
• Non-heterosexual dementia caregivers are
significantly younger and more likely to be employed
than heterosexual dementia caregivers and indicate
greater difficulty when paying for necessities while
also reporting higher family quality of life than their
heterosexual peers.
541
• Many caregivers of people with Alzheimer’s or other
dementias are at risk of social isolation.
542
Forty-one
percent of dementia caregivers in the 2014
Alzheimer’s Association poll reported that no one
else provided unpaid assistance.
A10
Caregiving

50 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
• Among dementia caregivers of care recipients who
have experienced severe psychiatric symptoms
(e.g., aggression, anxiety), those who live in low- or
medium-income neighborhoods indicate higher
distress than those caregivers living in high-income
neighborhoods.
543
• In a survey of caregivers from a large health care
system, less than 4 in 10 respondents (39.2%) agreed
that their primary care providers help them with
managing symptoms of a care recipient with
dementia.
544
Stress of Care Transitions
• Caregivers who helped someone with a formal
diagnosis of dementia indicated more emotional
difficulty and family disagreement than caregivers
of individuals without a formal diagnosis. However,
those caregivers of individuals with a formal dementia
diagnosis were also more engaged in communication
during doctors’ visits and also more likely to receive
caregiver training than those who assisted someone
without a diagnosis of dementia, suggesting the
importance of linking support to dementia diagnostic
procedures.
545
• Admitting a relative to a residential care facility has
mixed effects on the emotional and psychological
well-being of dementia family caregivers. Some
studies suggest that distress remains unchanged or
even increases for some caregivers (such as spouses),
but other studies have found that distress
decreases.
495, 546-548
• The demands of caregiving may intensify as people
with dementia approach the end of life.
549
In the year
before the death of the person living with dementia,
59% of caregivers felt they were “on duty” 24 hours
a day, and many felt that caregiving during this time
was extremely stressful.
550
The same study found
that 72% of family caregivers experienced relief
when the person with Alzheimer’s or another
dementia died.
550
• In the last 12 months of life, people with dementia
relied on more hours of family care (64.5 hours
per week) than people with cancer (39.3 hours
per week).
551
Caregiver Physical Health and Health Conditions
For some caregivers, the demands of caregiving may
cause declines in their own health. Evidence suggests
that the stress of providing dementia care increases
caregivers’ susceptibility to disease and health
complications.
552
As shown in Figure 10, page 48, 38% of
Alzheimer’s and other dementia caregivers indicate that
the physical stress of caregiving is high to very high.
A10
Dementia caregivers are 1.5 times more likely to indicate
substantial physical difficulty providing assistance to
their care recipients compared with non-dementia
caregivers.
553
The distress associated with caring for a
relative with Alzheimer’s or another dementia has also
been shown to negatively influence the quality of family
caregivers’ sleep.
554-557
Compared with those of the same
age who were not caregivers, caregivers of people with
dementia are estimated to lose between 2.4 hours and
3.5 hours of sleep a week.
555
Tables 10 and 11 present data on caregiver physical and
mental health. Table 10, page 51, presents state-by-state
data on the health status of dementia caregivers, and
Table 11, page 52, compares the percentages of dementia
caregivers, non-dementia caregivers and non-caregivers
who report having a specific chronic health condition.
General Health
Seventy-four percent of caregivers of people with
Alzheimer’s or other dementias reported that they were
“somewhat concerned” to “very concerned” about
maintaining their own health since becoming a
caregiver.
A10
A 2017 poll found that 27% of dementia
caregivers delayed or did not do things they should to
maintain their own health.
446, 558, 559
Data from the Health
and Retirement Study showed that dementia caregivers
who provided care to spouses were much more likely (41%
increased odds) than other spousal caregivers of similar
age to become increasingly frail during the time between
becoming a caregiver and their spouse’s death.
560-563
Physiological Changes
The chronic stress of caregiving may be associated
with an increased incidence of hypertension and a number
of physiological changes that could increase the risk of
developing chronic conditions, including high levels of stress
hormones, impaired immune function, slow wound healing
and coronary heart disease.
564-571
A recent meta-analysis
of studies examining the associations between family
caregiving, inflammation and immune function suggests
that dementia caregivers had slight reductions in immune
function and modestly elevated inflammation.
572
However,
a study of physiological changes before and after the start
of caregiving found no change in six biomarkers of
inflammation among dementia caregivers.
573
Health Care
When people with dementia also have depression,
behavioral disturbances or low functional status, their
caregivers face a higher risk of emergency department visits
and hospitalization compared with caregivers of people
with dementia without these challenges.
574, 575
Increased
depressive symptoms among caregivers are linked to more
frequent caregiver doctor visits, increased outpatient tests
and procedures, and greater use of over-the-counter and
prescription medications.
575
Dementia caregivers also have
twice the odds of experiencing an overnight hospitalization
than non-caregivers.
576

51Caregiving
*Data are for caregivers of individuals whose main reason for needing care is Alzheimer’s or other dementia.
For other states, the individuals' main reason for needing care could be another condition, but the individuals also
were living with Alzheimer's or other dementia.
†
Data not included because the sample size was less than 50 or the relative standard error was greater than 30%.
Created from data from the Behavioral Risk Factor Surveillance System Survey.
443
State
Percentage
Reporting at Least
One Chronic
Condition
Percentage
Reporting
Depression
Percentage
Reporting
Frequent
Poor Physical
Health
Alabama 57.5 30.9 15.0
Alaska 53.7 27.7 15.2
Arizona 66.7 27.7 15.5
Arkansas 72.8 38.0 25.0
California 61.0 18.6 13.1
Colorado 58.0 36.7 15.5
Connecticut 64.0 27.9 9.4
Delaware 61.8 23.3 †
District of Columbia* 65.1 † †
Florida 66.4 28.6 13.6
Georgia 64.9 33.2 15.1
Hawaii 49.6 16.5 8.1
Idaho 57.5 31.1 13.4
Illinois 64.2 29.0 †
Indiana 57.3 34.1 18.2
Iowa 60.5 27.4 13.8
Kansas 60.6 33.8 18.7
Kentucky 65.5 39.8 21.4
Louisiana 62.4 37. 2 15.9
Maine 60.8 38.0 12.8
Maryland 55.7 24.8 8.4
Massachusetts 54.2 20.2 †
Michigan 66.0 30.6 22.1
Minnesota 53.1 29.8 8.4
Mississippi 57.0 25.9 22.2
Missouri 59.5 28.1 20.2
State
Percentage
Reporting at Least
One Chronic
Condition
Percentage
Reporting
Depression
Percentage
Reporting
Frequent
Poor Physical
Health
Montana* 56.9 22.8 †
Nebraska 57.6 25.4 13.2
Nevada 54.2 31.1 †
New Hampshire 66.2 28.4 14.7
New Jersey 62.3 27.9 12.8
New Mexico 64.8 31.3 12.6
New York 59.0 24.7 12.0
North Carolina 58.8 41.0 18.1
North Dakota 60.1 30.4 8.6
Ohio 63.7 27.8 17.4
Oklahoma 68.2 39.6 17.2
Oregon 57.4 33.6 8.5
Pennsylvania 76.6 32.5 16.0
Rhode Island 54.2 41.0 11.5
South Carolina 60.6 31.0 15.2
South Dakota 61.0 22.2 †
Tennessee* 66.7 29.8 †
Texas 59.0 26.7 11.2
Utah 59.3 34.6 14.9
Vermont 61.5 35.4 10.7
Virginia 64.1 31.2 15.1
Washington 61.1 39.0 18.0
West Virginia 63.5 32.2 12.0
Wisconsin 62.9 27.8 18.9
Wyoming 59.8 22.8 †
Percentage of Dementia Caregivers Reporting Health Conditions by State, 2016-2022
Table
10

52
*Table includes caregivers age 18 and older.
†
Combination of coronary heart disease and stroke.
Created from data from the Behavioral Risk Factor Surveillance
System survey.
443
Mortality
Studies of how the health of people with dementia
affects their caregivers’ risk of dying have had mixed
findings.
577, 578
For example, spouses of hospitalized care
recipients with dementia were more likely to die in the
following year than caregivers whose spouses were
hospitalized but did not have dementia (after accounting
for differences in caregiver age).
579
In addition, caregivers
who perceived higher strain due to care responsibilities
were at higher risk for death than caregivers who
perceive little or no strain.
580
In contrast, a longitudinal
analysis of the Health and Retirement Study found that
dementia caregivers were less likely to die than non-
caregivers of similar age over a 12-year period. These
results are consistent with a protective effect of dementia
care, at least as it pertains to mortality.
577
The findings
are also consistent with the possibility that individuals
who assume dementia care roles do so in part because
their initial health allows them to do so. Eighteen percent
of spousal caregivers die before their partners with
dementia.
581
Caregiver Employment and Finances
Six in 10 caregivers of people with Alzheimer’s or
another dementia were employed or had been employed
in the prior year while providing care.
442
These
individuals worked an average of 35 hours per week
while caregiving.
442
Among people who were employed
in the past year while providing care to someone with
Alzheimer’s or another dementia, 57% reported
sometimes needing to go in late or leave early compared
with 47% of non-dementia caregivers. Eighteen percent
of dementia caregivers reduced their work hours due
to care responsibilities, compared with 13% of non-
dementia caregivers. In particular, adult daughters with
less than a high school degree are most likely to reduce
work hours when compared with other dementia
caregivers. Other work-related changes among dementia
and non-dementia caregivers who had been employed in
the past year are summarized in Figure 11.
442
In the 2018
National Health and Wellness Survey, close to 13% of
dementia caregivers in the United States indicated
absence from work in the past seven days due to a health
problem compared with 6% of dementia caregivers in
Japan and 10% of dementia caregivers across France,
Germany, Italy, Spain and the United Kingdom.
448
In
addition, caregivers living with a family member with
dementia pay for 64% of total care costs (e.g., total health
care spending and out-of-pocket costs) incurred during
their relatives' last seven years of life.
582
In 2021, it was estimated that dementia caregivers bore
nearly twice the average out-of-pocket costs of non-
dementia caregivers ($12,388 versus $6,667).
431, 583
Examples include costs of medical care, personal care and
household expenses for the person with dementia, and
personal expenses and respite services for the caregiver.
Caregivers of a spouse with dementia indicate higher
home health care expenditures but lower outpatient
expenditures than those who do not have a spouse with
dementia, which suggests a possible “substitution” effect
and greater referrals to home health care by providers for
patients with dementia.
584, 585
National survey data among
“care contributors” (or, a friend or relative who paid for
dementia expenses and/or provided care for someone
with dementia at least once a month in the prior year)
revealed that 48% cut back on other spending and 43%
cut back on savings due to the out-of-pocket costs of
providing help to someone with dementia.
513
Due to care
responsibilities, close to 4 in 10 care contributors
indicated that the “food they bought just didn’t last, and
they didn’t have money to get more,” and 3 in 10 ate less
because of care-related costs.
513
One in 5 caregivers of people with Alzheimer’s or other
dementias (22%) report problems dealing with a bank
or credit union when helping to manage the finances
of people living with dementia, compared with 9% of
caregivers of people without dementia.
442
Condition
Dementia
Caregivers
Non-
Dementia
Caregivers
Non-
Caregivers
Stroke 5.2 3.4 3.2
Coronary heart disease 8.3 7.2 6.6
Cardiovascular disease
†
11.8 9.5 8.6
Diabetes 12.8 11.1 11.3
Cancer 14.3 13.3 11.5
Obesity 32.7 34.6 29.5
Percentage of Dementia Caregivers Who Report Having
a Chronic Health Condition Compared with Caregivers of
People without Dementia or Non-Caregivers*
Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Table
11

53
Created from data from the National Alliance for Caregiving in Partnership with the Alzheimer’s Association.
442
Effects of Stress and Other Caregiving Factors on
People with Dementia
Research has documented the effects of caregiver stress
on people with dementia and their use of health care
services. For example, distress on the part of family
caregivers is associated with increased odds of residential
care entry for the person with dementia, exacerbated
behavioral and psychological challenges in the person with
dementia, and increased likelihood of someone with
dementia being abused.
586
Individuals with dementia are
more likely to be hospitalized if their caregiver has less
than one year of caregiving experience when compared
with caregivers who have provided assistance for more
than one year.
587
In addition, care recipients with dementia
whose caregivers indicate greater distress are also more
likely to experience hospitalization.
587, 588
A synthesis of
available qualitative studies found that “personhood,” or
the extent to which others value, support and establish
meaningful relationships with someone with dementia, is
enhanced through personal interactions with family,
friends, other people with dementia and professional
caregivers as well as through opportunities for ongoing
engagement in social and occupational activities/roles.
589
Some meta-analyses suggest that care coordination/case
management and psychoeducational and multi-component
programs delivered to dementia caregivers may improve
important care recipient outcomes, including improvements
in behavior, mood and quality of life and delayed
institutionalization. However, effects sizes are small.
590
Interventions Designed to Assist Caregivers
For more than 35 years, strategies to support family
caregivers of people with dementia have been developed
and evaluated. The types and focus of these strategies (often
called “interventions”) are summarized in Table 12.
511, 591
In general, the goal of interventions is to improve the
health and well-being of dementia caregivers by relieving
the negative aspects of caregiving. Some also aim to delay
nursing home admission of the person with dementia by
providing caregivers with skills and resources (emotional,
social, psychological and/or technological) to continue
helping their relatives or friends at home. Specific
approaches used in various interventions include
providing education to caregivers, helping caregivers
manage dementia-related symptoms, improving social
support for caregivers and providing caregivers with
respite from caregiving duties.
According to a publication on dementia caregiver
interventions that reviewed seven meta-analyses and
17 systematic reviews of randomized controlled trials,
the following characteristics distinguish interventions
Caregiving
Caregivers of people with Alzheimer’s or other dementiasPercentage Caregivers of other people
60
50
40
30
20
10
0
57%
47%
18%
13%
16%
14%
9%
5%
8%
4%
7% 7% 7%
2%
6%
4%
Went in late,
left early or
took time off
Took a leave
of absence
Turned down
a promotion
Lost any
benefits
Changes Went from full-
to part-time or
cut back hours
Gave up
working entirely
Received a warning
about performance/
attendance
Retired
early
Work-Related Changes Among Caregivers of People with Alzheimer’s or Other
Dementias Who Had Been Employed at Any Time Since They Began Caregiving
Figure
11

that are effective: family caregivers are actively involved
in the intervention, in contrast to passively receiving
information; the intervention is tailored and flexible to
meet the changing needs of family caregivers during the
course of a relative’s dementia; and the intervention
meets the needs not only of caregivers but of people
living with dementia as well.
592
A meta-analysis examining
the components of dementia caregiver interventions that
are most beneficial found that interventions that initially
enhance caregiving competency, gradually address the
care needs of the person with dementia, and offer
emotional support for loss and grief when needed
appeared most effective.
593
A prior report examined
randomized, controlled studies of caregiver interventions
and identified 44 interventions that benefited individuals
with dementia as well as caregivers, and more such
interventions are emerging each year.
594-599
Although
several national reports have suggested that the available
scientific evidence does not provide clear suggestions as
to which intervention types benefit dementia caregivers
consistently,
600
other recent meta-analyses report that
specific intervention types (such as psychoeducation;
see Table 12) may result in a small reduction in burden for
caregivers, with other meta-analyses indicating broader
effects of various interventions across multiple dementia
caregiver outcomes.
590, 601-605
A meta-review of over 60
meta-analyses and systematic reviews of dementia
caregiver interventions indicate that although various
interventions may have positive effects on depression and
other measures of caregiver well-being, challenges
related to how interventions are reported and classified
has made it difficult to ascertain what works and why for
dementia caregivers.
606
Interventions for dementia caregivers that have
demonstrated efficacy in scientific evaluations have been
gradually implemented in the community, but are still not
widespread or available to all family caregivers.
607-609
When interventions are implemented, they are generally
successful at improving how caregiver services are
delivered and have the potential to reach a large number
of families while also helping caregivers cope with their
responsibilities (this includes the Alzheimer’s Association
24/7 Helpline).
610-613
In one example, researchers utilized
an “agile implementation” process to more rapidly select,
locate, evaluate and replicate a collaborative care model
for dementia care. This care model has successfully
operated for over a decade in an Indianapolis health care
system.
614
Other efforts have attempted to broaden the
reach and accessibility of interventions for dementia
caregivers through the use of technologies (for instance,
video-phone delivery and online training),
615-623
while
others have disseminated evidence-based dementia care
Type Focus
Case management Provides assessment, information, planning, referral, care coordination and/or advocacy for
family caregivers.
Psychoeducational
approaches
Include structured programs that provide information about the disease, resources and services, and about
how to expand skills to effectively respond to symptoms of the disease (for example, cognitive impairment,
behavioral symptoms and care-related needs). Include lectures, discussions and written materials and are led
by professionals with specialized training.
Counseling Aims to resolve preexisting personal problems that complicate caregiving to reduce conflicts between
caregivers and care recipients and/or improve family functioning.
Psychotherapeutic
approaches
Involve the establishment of a therapeutic relationship between the caregiver and a professional therapist
(for example, cognitive behavioral therapy for caregivers to focus on identifying and modifying beliefs
related to emotional distress, developing new behaviors to deal with caregiving demands, and fostering
activities that can promote caregiver well-being).
Respite Provides planned, temporary relief for the caregiver through the provision of substitute care; examples
include adult day services and in-home or institutional respite care for a certain number of weekly hours.
Support groups Are less structured than psychoeducational or psychotherapeutic interventions. Support groups provide
caregivers the opportunity to share personal feelings and concerns to overcome feelings of isolation.
Multicomponent
approaches
Are characterized by intensive support strategies that combine multiple forms of intervention, such as
education, support and respite, into a single, long-term service (often provided for 12 months or more).
Type and Focus of Caregiver Interventions
Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).54
Created from data from Sörensen et al.,
511
Gaugler et al.
591
and Walter and Pinquart.
603
Table
12

55Caregiving
COVID-19 and Dementia Caregiving
Existing reports indicate that the COVID-19 pandemic
has posed significant social, psychological, emotional
and physical challenges to family members and friends
who provide care for people with dementia.
657-667
Fatigue and burnout among dementia caregivers and
their lack of access to services and supports for
themselves and for the people for whom they provide
care are common themes in research on the wide-
ranging effects of the COVID-19 pandemic.
668
For
these reasons the pandemic has created a crisis for
dementia caregivers.
657, 669, 670
Telephone interviews with family caregivers in rural
Virginia following the governor's stay-at-home order
in 2020 found that those who were more concerned
about the COVID-19 pandemic and those who received
less help from family and friends experienced greater
feelings of emotional exhaustion and fatigue related to
dementia care.
671
In the earlier stages of the pandemic,
caregivers were limited in or completely barred from
visiting and communicating with relatives who lived in
long-term care residences due to COVID-19 lockdown
procedures. The inability to visit or engage with relatives
resulted in distress as well as significant concerns about
the health of relatives living in residential long-term
care during the pandemic.
672, 673
Studies of end-of-life
care during the pandemic indicated that dementia
caregivers felt that enforced social isolation was
prevalent and adversely influenced the death and dying
experience of relatives during the pandemic.
674
Adult
day programs and other community-based services in
many states were interrupted or closed.
675
These and
other factors shaped by the COVID-19 pandemic have
caused emotional distress and other negative outcomes
among caregivers.
623, 676
In addition, staff and directors
of adult day service programs in the United States
reported perceived declines in cognition, function and
well-being among clients due to state closures during
the pandemic.
676
Together, this suggests the need for
improved support of long-term programs that serve
community-residing people with dementia and their
caregivers as well as strategies/policies to maintain links
between family caregivers and residents of congregate
care settings (assisted living, nursing homes) during
future public health emergencies.
677, 678
Studies have shown that family caregivers who were
able to engage in more direct phone and email contact
with relatives in long-term care residences during
COVID-19 lockdowns indicated greater emotional
well-being for themselves and their relatives, whereas
relying on residential care staff to engage in
communication resulted in lower perceived well-being
among family caregivers and their relatives.
679
Other
studies suggested that some dementia family caregivers
adjusted during the pandemic by relying more heavily
on other sources of family/unpaid help as well as
technologies to maintain social connection with
relatives.
680-683
In studies of dementia caregivers of
relatives living in nursing homes or similar residential
settings, caregivers indicated a number of challenges
during the COVID-19 pandemic, including severely
limited contact with relatives due to visitation
restrictions, a lack of transparent information and
communication from care residences, fears of relatives
dying alone and concerns about overburdened staff at
care residences.
673, 684
In addition, caregivers highlighted
a number of resources and practices that were helpful
during COVID-19, including effective infection control
measures adopted by care residences, robust
communication with staff, and the need for creativity
when remaining socially connected with relatives in
nursing homes or similar residential settings.
673
There
is also evidence of racial and gender differences in
dementia care provision during the pandemic. Compared
with White dementia and non-dementia caregivers as
well as Black non-dementia caregivers, Black dementia
caregivers provided greater ADL care to relatives with
dementia.
456
Providing telehealth support to dementia
caregivers that was culturally appropriate, delivering
COVID-19 safety education, and offering compassionate
listening appeared to benefit social connections and
reduce distress.
685, 686
In a survey, women dementia
caregivers were more likely to indicate needs related
to carrying out caregiving responsibilities during the
pandemic, whereas men indicated more needs for health
and social resources. Men were also more likely to
report psychological distress.
687
At the outset of the pandemic, the National Institutes
of Health and other federal agencies issued multiple
requests for rapid grant applications to study and design
interventions to mitigate the effects of COVID-19 on
people with dementia and their caregivers.
688
The
Alzheimer’s Association also provided regularly updated
guidance for dementia caregivers and professional care
providers as the pandemic unfolded. In addition, the
challenges of the pandemic have motivated some
service providers to transition their support programs
toward remote/virtual care delivery, which has helped to
extend the reach and accessibility of dementia care
innovations.
689, 690
Concerns remain, however, about the
“digital divide” facing caregivers who do not have reliable
broadband access or do not regularly use the internet.

56 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
interventions into community-based programs and health
care systems.
610, 624, 625
Dissemination efforts, such as
Best Practice Caregiving, have attempted to provide
tools and resources to providers and others to facilitate
the implementation of successful interventions into
community-based organizations, health care systems
and other “real-world” settings.
626
Because caregivers and the settings in which they
provide care are diverse, more studies are required to
define which interventions are most effective for
specific situations and how these interventions are
successful.
627-631
Improved tools and measures to
personalize services for caregivers to maximize their
benefits represent an emerging area of research.
632-637
More studies are also needed to adapt proven
interventions or develop new intervention approaches for
families from different racial, ethnic and socioeconomic
backgrounds and in different geographic settings.
462, 638-646
Additional research on interventions focused on disease
stages is also required, as is research on specific
intervention needs for LGBT caregivers for whom a lack
of inclusive practices on the part of health care
professionals, stigma, and a reluctance to seek support
may result in greater unmet needs compared with
non-LGBT dementia caregivers.
439, 647, 648
In 2019, the National Institute on Aging (NIA) awarded
funding to create the NIA Imbedded Pragmatic AD/ADRD
Clinical Trials (IMPACT) Collaboratory. The Collaboratory
includes experts from more than 30 research universities/
centers and supports pilot trials and larger studies that
test non-drug, care-based interventions for people living
with dementia. The goal of IMPACT is to expedite the
timeline of research implementation in real-world
settings to improve care for people living with dementia
and their caregivers. In 2020, the CDC established three
Public Health Centers of Excellence on dementia to
disseminate best practices and tools to local, tribal and
state public health organizations throughout the
United States; one of those Centers focuses on
dementia caregiving.
The Alzheimer’s Association has also undertaken several
efforts to improve dementia care interventions and
services. Its dementia care practice recommendations
649
place individuals with dementia and their caregivers at the
center of how care should be delivered (see Figure 12).
Essential to this model is the need to reconsider how care
for people with dementia is measured and designed by
moving away from an approach that focuses on loss of
abilities to one that emphasizes the individual’s unique
needs, personal experiences and strengths. This person-
centered care philosophy not only values and respects the
individual with dementia but also promotes well-being and
health.
589, 650
Frameworks such as the Alzheimer’s
Created from data from the Alzheimer’s Association.
649
Association dementia care practice recommendations
are designed to shift how researchers and care providers
think about dementia and may point the way to a
greater understanding of the resilience, adaptability and
possibilities of maintenance or even improvement of
skills and abilities when living with dementia.
651, 652
A core
element of these frameworks is ensuring that every
experience and interaction is seen as an opportunity to
have authentic and meaningful engagement, which in
turn helps create a better quality of life for the person
with dementia and their caregivers.
Trends in Dementia Caregiving
There is some indication that families have greater
capacity to manage the care they provide to relatives
with dementia than in the past. Compared with dementia
caregivers in 1999, dementia caregivers in 2015 were
significantly less likely to report physical difficulties (from
30% in 1999 to 17% in 2015) and financial difficulties
(from 22% in 1999 to 9% in 2015) related to care
provision. In addition, use of respite care by dementia
caregivers increased substantially (from 13% in 1999 to
27% in 2015).
499
However, as noted earlier, more work is
needed to ensure that interventions for dementia
caregivers are available and accessible to those who need
them. A 2016 study of the Older Americans Act’s National
Family Caregiver Support Program found that over half
Individuals with
Dementia and
Their Caregivers
Detection and
Diagnosis
Assessment and
Care Planning
Medical
Management
Information,
Education and
Support
Dementia-
Related
Behaviors
Activities of
Daily Living
Workforce
Supportive and
Therapeutic
Environment
Transition and
Coordination
of Services
Person-Centered Care Delivery
Figure
12
57
(52%) of Area Agencies on Aging did not offer evidence-
based family caregiver interventions.
653
In addition, there
is some indication that the number of family members
available to provide care to older relatives with
health needs is likely to decrease due to a range of
sociodemographic and health trends in the U.S. (e.g., the
aging of the U.S. population, a lower birth rate and adult
children’s geographic mobility/dispersion over the prior
several decades).
654
The need to bridge this impending
“family care gap” and other dementia caregiving
challenges and concerns through new policies, services
and research is a growing public health concern.
655, 656
A National Strategy to Support
Family Caregivers
The Recognize, Assist, Include, Support, and Engage
(RAISE) Family Caregivers Act, which was signed into law
in January 2018, authorized the Secretary of Health
and Human Services to develop the first national
strategy to support family caregivers. To advance the
development of this strategy, a 30-member Family
Caregiving Advisory Council was established to provide
key recommendations, guidance and best practices that
support family caregivers. In September 2022 the
Advisory Council delivered its National Strategy to
Support Family Caregivers to Congress. It features
nearly 350 actions that 15 federal agencies will adopt
and 150 actions that states, communities and others can
take. The four core principles that drive these many
supportive actions include: 1) placing the family and
person at the center of all interactions; 2) addressing
trauma and its impact on families; 3) advancing equity,
accessibility and inclusion for family caregivers in
underserved communities; and 4) elevating direct care
workers as family caregiving partners.
691
On July 31, 2023, the Centers for Medicare & Medicaid
Services (CMS) announced that beginning July 2024
it will support the Guiding an Improved Dementia
Experience (GUIDE) Model until 2032. The GUIDE
Model features the provision of comprehensive
dementia care coordination and management, caregiver
education and support, and respite services. Individuals
living with dementia and their caregivers will also have
access to a 24/7 support line. The GUIDE Model is
unique in that it incentivizes providers to incorporate
both the person with dementia and the caregiver
(or caregivers) into the collaborative, multidisciplinary
service approach. Critically, CMS will include policies
to ensure that underserved communities have equal
access to GUIDE Model services to address disparities
in access to and quality of dementia care.
692
Caregiving

Workforce
More than 1 million additional
direct care workers will be needed
between 2021 and 2031 — more
new workers than in any other single
occupation in the United States.
59
As the prevalence of Alzheimer’s disease and other
dementias increases, so does the need for more members
of the paid workforce to be knowledgeable and skillful
about working with a diverse population of people living
with dementia as well as with their families.
693, 694
A dementia-capable workforce addresses the
full arc of care — from identifying a concern
to screening, detecting and diagnosing within
clinical settings, to treating, monitoring and
caring for those living with these diseases in
residential or home and community-based
settings. This workforce includes, but is not
limited to, primary care physicians (PCPs) and
advanced practice clinicians; specialists, such
as geriatricians, neurologists and psychiatrists;
other licensed providers, such as registered
nurses, psychologists, therapists and social
workers; members of the direct care workforce,
including personal care aides, home health
aides and nursing assistants; and the broader
community-based workforce who interact
with the public and help meet the needs of
people living with dementia, such as police
officers, bank tellers, librarians, hairdressers,
bus drivers and others.
Screening, Detecting and Diagnosing
Workforce
Improving dementia screening, detection and diagnosis
is a high priority.
267, 695-697
A recent study of Medicare
beneficiaries found that only about 8% of expected mild
cognitive impairment (MCI) cases are diagnosed on
average, suggesting there may be as many as 8 million
people with undiagnosed MCI (acknowledging that not
all individuals with MCI develop dementia; see Overview,
page 4).
271, 698
Among over 200,000 clinicians and
practices surveyed, only 0.1% had diagnosis rates within
the expected range, likely due to limited expertise and
confidence and other factors discussed on the following
pages.
271
See the Special Report from 2022 Alzheimer’s
Disease Facts and Figures that examines consumers’ and
primary care physicians' perspectives on awareness,
diagnosis and treatment of MCI, including MCI due to
Alzheimer’s disease.
697
With early detection of cognitive impairment comes
opportunities for individuals and their families to plan for
future care and to participate in clinical trials or be
treated with FDA-approved disease-modifying therapies.
Suboptimal detection and diagnosis of dementia,
conversely, reduces the ability of individuals and their
families to make informed decisions, access appropriate
medical care, create financial and legal plans, and pursue
services and support. Timely and accurate detection is
particularly important considering that dementia is
progressive. While more evidence is needed to support
screening of asymptomatic individuals,
699
it is generally
accepted that clinically significant cognitive concerns that
arise in the primary care setting should be followed by an
evaluation for cognitive impairment using a standardized
and validated assessment.
700-702
See the Special Report
from 2019 Alzheimer’s Disease Facts and Figures that
explores the state of cognitive assessment in the primary
care setting and identifies potential solutions for existing
barriers to widespread adoption of assessment in primary
care settings.
267
Health care professionals who are involved in screening
for, detecting, and/or diagnosing Alzheimer’s and other
dementias include PCPs (e.g., family medicine and internal
medicine physicians), advanced practice clinicians (e.g.,
nurse practitioners and physician assistants), and specialists
such as geriatricians (who specialize in caring for older
adults), neurologists (especially geriatric and cognitive
neurologists), neuropsychologists, geropsychologists and
geriatric psychiatrists. However, limited skill and confidence
in diagnosing dementia,
703, 704
time constraints among
PCPs during routine office visits
705, 706
and a widespread
shortage of geriatricians and other specialists has resulted
in delayed screening, detection and diagnosis of Alzheimer’s
disease and other dementias.
Workforce

Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).60
The 10% column is how many geriatricians will be needed to serve only those 65 and older projected to have Alzheimer’s dementia in 2050,
assuming that the percentage of people age 65 and older with Alzheimer’s dementia remains at approximately 10%. The 30% column is how many
geriatricians will be needed to serve the 30% of people age 65 and older who need geriatrician care, regardless of whether they have dementia.
The number of practicing geriatricians in 2021 was provided by IQVIA and includes physicians with geriatrics as either their primary or secondary
specialty. Calculations assume that each geriatrician can care for up to 700 patients.
725
The underlying state-by-state estimates of the 2050
population age 65 and older were provided by Claritas Pop-Facts 2020.
Number of Geriatricians in 2021 and Projected Number of Geriatricians Needed in 2050 by State
Montana 9 59 177
Nebraska 23 84 253
Nevada 43 158 474
New Hampshire 33 72 217
New Jersey 206 398 1,193
New Mexico 27 93 279
New York 568 818 2,454
North Carolina 158 535 1,606
North Dakota 12 34 103
Ohio 163 537 1,611
Oklahoma 26 171 512
Oregon 69 232 695
Pennsylvania 273 601 1,803
Rhode Island 33 49 147
South Carolina 66 288 865
South Dakota 15 44 131
Tennessee 37 343 1,029
Texas 333 1,255 3,766
Utah 25 114 341
Vermont 9 32 95
Virginia 113 406 1,218
Washington 126 399 1,198
West Virginia 16 83 250
Wisconsin 83 273 820
Wyoming 3 26 79
U.S. Total 5,170 15,417 46,252
Alabama 33 228 684
Alaska 8 31 92
Arizona 92 363 1,089
Arkansas 55 134 402
California 587 1,676 5,029
Colorado 96 289 867
Connecticut 91 166 497
Delaware 18 55 165
District of Columbia 36 28 83
Florida 362 1,365 4,096
Georgia 100 492 1,476
Hawaii 63 64 192
Idaho 8 87 261
Illinois 212 517 1,551
Indiana 66 299 897
Iowa 26 142 426
Kansas 20 121 364
Kentucky 39 207 622
Louisiana 31 198 595
Maine 36 71 213
Maryland 146 288 865
Massachusetts 214 347 1,042
Michigan 164 465 1,394
Minnesota 84 270 811
Mississippi 23 124 373
Missouri 91 283 849
Number of Number of
Geriatricians Geriatricians
Number of Needed in 2050 Needed in 2050
Geriatricians to Serve 10% of to Serve 30% of
State in 2021 Those 65 and Older Those 65 and Older
Number of Number of
Geriatricians Geriatricians
Number of Needed in 2050 Needed in 2050
Geriatricians to Serve 10% of to Serve 30% of
State in 2021 Those 65 and Older Those 65 and Older
Table
13
61
Primary Care Physicians
PCPs are most likely to make the initial diagnosis of
dementia.
707, 708
A study of Medicare beneficiaries found
that 85% of people living with dementia were diagnosed by
providers who do not specialize in dementia (e.g., PCP,
emergency medicine physician, nurse practitioner, clinical
psychologist). Among the remaining 15% diagnosed by a
provider who specializes in dementia, 47% were diagnosed
by a psychiatrist, including geriatric psychiatrists and
neuropsychiatrists, 44% by a neurologist and 9% by
a geriatrician.
709
PCPs are well-situated to detect dementia because they
often have long-standing relationships with patients and
may witness clinical manifestations of cognitive decline —
both overt functional and communication changes and
subtle signs, such as irregularities in medication or
appointment adherence, loss of control of chronic disease,
weight loss, or increase in emergency room visits or
hospitalizations. Even though the vast majority of initial
dementia diagnoses are made by PCPs, studies have found
that diagnosis is delayed until moderate or advanced stages
in 50% or more patients with Alzheimer’s,
710, 711
with greater
delays among individuals from racial and ethnic minority
groups.
712-715
It is important to reiterate that many people
living with dementia never receive a diagnosis in the
primary care setting (see Prevalence section). During these
delays or lack of diagnosis, people living with dementia
could otherwise have been enrolled in potentially life-
changing clinical trials of new treatments, begun taking
currently approved treatments, receiving emotional
support through a support group of others living with
dementia, and started planning for financial, caregiving and
accommodation changes they may experience as their
condition progresses.
If a person shows signs of cognitive impairment during a
routine doctor’s visit, Medicare covers a separate visit to
assess the person’s cognitive function and develop a care
plan.
716, 717
As of January 1, 2024, Medicare reimbursed
approximately $268 to physicians and other eligible billing
practitioners for providing a comprehensive clinical visit
that results in a written care plan (current procedural
terminology code 99483; rate may be geographically
adjusted).
716, 718-720
Although screening is now a
reimbursable service by Medicare,
716
PCPs experience
numerous barriers to detecting cognitive impairment and
diagnosing dementia.
267
For instance, commonly used
cognitive assessments take time and training to administer,
interpret, document and follow up on, which makes them
hard to use in a busy practice setting.
704, 721
Furthermore,
the next steps following detection can be seen as a barrier
as many PCPs report low confidence disclosing a dementia
diagnosis and providing post-diagnostic care.
704, 722
Even if
dementia is diagnosed, providers sometimes wait to
disclose this information to the patient due to diagnostic
uncertainty, time constraints, stigma and fear of causing
emotional distress. The U.S. Government Accountability
Office (GAO) found that use of the cognitive assessment
and care plan service in traditional fee-for-service
Medicare tripled from 2018 through 2022. However, use of
the service was relatively low among Medicare beneficiaries
diagnosed with a cognitive impairment; GAO estimated that
in 2021, the most recent year of data available, only 2.4% of
beneficiaries with a dementia diagnosis received the service
through traditional Medicare.
723
Among PCPs surveyed by the Alzheimer’s Association in
2019, nearly 40% reported that they were “never” or “only
sometimes” comfortable making a diagnosis of Alzheimer’s
or another dementia.
703
More than 25% of PCPs reported
being “never” or “only sometimes” comfortable answering
patient questions about Alzheimer’s or other dementias,
and 50% did not feel adequately prepared to care for
individuals who had been diagnosed. Given this discomfort
and uncertainty, almost one-third of PCPs in the survey
reported referring patients to specialists; however, most
PCPs (55%) reported that there were not enough
specialists (e.g., geriatricians) in their area to meet the
demand.
703
See the Special Report from 2020 Alzheimer’s
Disease Facts and Figures that examines the gaps and
projected shortages in specialty care for Alzheimer’s and
other dementias.
724
Geriatricians and Other Specialists
There is a particular need for geriatricians to screen for,
detect, and diagnose possible dementia. Geriatricians are
family physicians or board-certified internists who are
specially trained to evaluate and manage the unique health
care needs and treatment preferences of older adults.
Up to 30% of people age 65 and older are estimated to
need a geriatrician.
725
There were approximately 5,170 to
7,454 geriatricians in the United States in 2021, depending
on the source of the estimate,
726, 727
indicating a sizable
and potentially consequential shortage relative to need.
Indeed, the National Center for Health Workforce Analysis
(NCHWA) determined that there was already a shortage of
geriatricians a decade ago,
728
and the projected increase in
demand for geriatricians by 2050 is expected to far exceed
the supply in every region of the United States.
725, 728, 729
Table 13 shows state-by-state projections for the number
of geriatricians needed in 2050, using December 2021
data from IQVIA as a starting point.
727
If things continue at
the current pace, the United States will have to nearly
triple the number of geriatricians who were practicing in
2021 to effectively care for the approximately 10% of
those 65 and older who are projected to have Alzheimer's
dementia in 2050. The number must increase nearly nine
Workforce
Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
times to have enough geriatricians to care for the
approximately 30% of the population age 65 and older who
will need geriatrician care.
725
These shortages will affect states differently. For example,
Hawaii and Washington, D.C., have almost enough or more
than enough geriatricians (respectively) to match the
approximately 10% of those age 65 and older projected to
have Alzheimer’s dementia in 2050. In contrast, 12 states
need to at least quintuple the number of practicing
geriatricians by 2050 to care for those 65 and older
projected to have Alzheimer’s dementia, or increase the
number by at least 13 times to care for the 30% of the
population age 65 and older projected to need geriatrician
care. Two states, Tennessee and Idaho, will need to increase
the number of geriatricians by at least nine times just to
meet the care needs of those projected to have
Alzheimer’s dementia in 2050, or by at least 29 times to
meet the needs of all those age 65 and older who are
projected to need geriatrician care in 2050.
While the shortage of geriatricians and other specialists
extends nationwide, it appears to be most acute in rural
settings — with many rural counties facing a shortage of
health care providers overall.
730, 731
For instance, according to
the 2019 Alzheimer’s Association survey, 44% of PCPs in
large cities and 54% in suburban areas near large cities
reported that there were not enough specialists in their
area, while 63% of PCPs in small cities or towns and 71%
of PCPs in rural areas reported the same.
703
Another
indicator of the growing shortage of geriatricians is that,
in 2023, there were 411 geriatric medicine fellowship
positions available, but more than half (234) went unfilled.
732
The shortage of specialists extends to neurologists as well.
The National Center for Health Workforce Analysis
(NCHWA) projected that there will be a shortfall of
neurologists by 2025, but suggested that the growing
number of physician assistants in neurology could help
address this workforce gap.
733
Twenty U.S. states have
already been identified as “dementia neurology deserts,”
meaning they are projected to have fewer than 10
neurologists per 10,000 people with dementia in 2025.
734
The shortage of geriatricians and other relevant specialists
has been attributed to a combination of factors, including
growth in demand due to population aging; a smaller
percentage of working aged adults; substantively lower pay
for geriatricians and neurologists compared with many other
specialist physicians; an inadequate number of clinician
educators with relevant specialties on the faculties of health
professional schools; limited availability of incentives to
choose these specialties, such as loan forgiveness programs;
and the insufficient respect and recognition accorded to
geriatricians and related specialists.
735, 736
Many of these
factors are modifiable and must be addressed in order to
increase the number of providers available to provide
specialized dementia diagnosis and care. Moreover, beyond
increasing the supply of dementia specialists, geriatric care
principles should be integrated across all health care
professionals' educational curricula to ensure widespread
delivery of age-friendly care.
Medical Treatment and Care Team Workforce
As well as screening for, detecting and diagnosing
dementia, PCPs are responsible for managing treatment
for people living with dementia.
737
Even so, dementia care
is inadequately covered in health care training programs,
both in curricula and in opportunities for clinical practice.
738
Advanced practice providers, including physician
assistants and nurse practitioners, also play key roles in
treatment for people living with dementia. However,
there is limited specialization in caring for older adults
among these advanced practice roles, likely for many of
the same reasons cited for the shortages of geriatricians
and neurologists. In 2022, there were 355,000 nurse
practitioners licensed in the United States, up from 91,000
in 2010, but less than 1% had a certification in gerontology,
6.1% had a certification in gerontology acute care, and
8.9% had a certification in gerontology primary care.
739, 740
Also, less than 700 geriatric psychiatrists were certified by
the American Board of Psychiatry and Neurology (ABPN)
in the last decade (from 2012 to 2020)
741
and over half of
geriatric psychiatrists certified by the ABPN are
concentrated in just seven states (2015).
742
As of 2018, less than 1% of physician assistants were
certified in geriatric medical care. Although this figure
represents a nearly 400% increase in the absolute number
of physician assistants specializing in geriatric care since
2013 (indicating a positive trend),
743
significant gaps remain
in the capacity of this workforce to support older adults
living with dementia and other chronic health concerns.
Registered nurses, licensed practical nurses, licensed
psychologists and licensed therapists comprise other
critical segments of the dementia care workforce,
providing a range of nursing, rehabilitation and supportive
services in community settings, skilled nursing homes and
other settings. These services include medication
administration, intravenous injections, wound care,
catheter care, physical therapy, occupational therapy,
behavioral consultation and much more. In addition, social
workers assist with care navigation and management, and
licensed clinical social workers and psychologists may
provide therapeutic services to people living with dementia
and their caregivers.
Specialization in caring for older adults, however, remains
limited across these occupational groups as well. For
instance, a survey of Masters of Social Work students who
62

63Workforce
Created from data from Projections Managing Partnership. Projections Central: Long-Term Occupational Projections (2020-2030).
Available at: https://www.projectionscentral.org/Projections/LongTerm. Accessed January 17, 2024.
Expected Home Health and Personal Care Aide Job Growth, 2020-2030
Percentage
Increase
State 2020 2030 2020-2030
Alabama 21,700 25,910 19.4
Alaska 6,270 7,130 13.7
Arizona 72,920 117,740 61.5
Arkansas 21,900 28,350 29.5
California 766,000 985,800 28.7
Colorado 36,890 49,220 33.4
Connecticut 44,180 53,250 20.5
Delaware 8,430 11,780 39.7
District of Columbia 12,120 15,180 25.2
Florida 76,140 93,270 22.5
Georgia 44,060 60,350 37.0
Hawaii 9,290 12,270 32.1
Idaho 17,400 20,640 18.6
Illinois 99,460 118,600 19.2
Indiana 42,200 55,720 32.0
Iowa 23,880 31,580 32.2
Kansas 25,710 30,110 17.1
Kentucky 22,230 30,130 35.5
Louisiana 37,900 44,160 16.5
Maine 17,380 18,710 7.7
Maryland 42,560 56,790 33.4
Massachusetts 109,430 139,560 27.5
Michigan 71,750 89,820 25.2
Minnesota 107,500 133,420 24.1
Mississippi 19,130 25,200 31.7
Missouri 75,960 86,160 13.4
Percentage
Increase
State 2020 2030 2020-2030
Montana 7,190 9,670 34.5
Nebraska 12,500 15,210 21.7
Nevada 15,830 23,860 50.7
New Hampshire 8,410 10,970 30.4
New Jersey 59,610 76,930 29.1
New Mexico 32,360 40,750 25.9
New York 510,870 710,570 39.1
North Carolina 65,150 82,070 26.0
North Dakota 6,790 8,540 25.8
Ohio 95,560 118,540 24.0
Oklahoma 20,460 26,210 28.1
Oregon 32,330 39,960 23.6
Pennsylvania 175,140 214,740 22.6
Rhode Island 7,410 9,450 27.5
South Carolina 31,750 41,850 31.8
South Dakota 3,830 4,570 19.3
Tennessee 31,470 44,740 42.2
Texas 320,780 418,500 30.5
Utah 17,080 22,440 31.4
Vermont 7,770 10,310 32.7
Virginia 56,390 73,160 29.7
Washington 63,300 80,760 27.6
West Virginia 16,470 21,370 29.8
Wisconsin 77,810 92,320 18.6
Wyoming 3,750 5,020 33.9
U.S. Total 3,470,700 4,600,600 32.6
Number in 2020
and Projected Number
Needed in 2030
Number in 2020
and Projected Number
Needed in 2030
Table
14
64 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
graduated from 2017 to 2019 found that only 4.2% were
specializing in aging or gerontology.
744
Despite these low
percentages, between 20% to 48% of social work students
have high interest in working with older adults.
745-749
Since
the social work profession has a broad scope, student
interest may focus on social problems rather than age-based
populations.
750
Other reports indicate that less than 1% of
registered nurses are certified in geriatrics
751, 752
and only
1.2% of psychologists specialize in geropsychology.
753
Collaborative Workforce Models for
Dementia Care Management
Several decades of research supports the value of
collaborative primary care models that bring different health
professionals together, such as social workers, registered
nurses and non-clinical care managers, in addition to
physicians and advanced practice providers, to care for
people living with dementia.
754, 755
Researchers and
practitioners have identified eight essential elements of
comprehensive dementia care to improve outcomes and
lower costs: treatment of related conditions, coordination of
care, continuous monitoring and assessment, ongoing care
plan, psychosocial interventions, self-management, caregiver
support and medication management.
756
These collaborative
and comprehensive models have been associated with a
range of benefits including reduced behavioral symptoms,
improved function and quality of life, decreased caregiver
burden, and lower health care costs related to
hospitalizations, emergency department visits and other
outpatient visits.
755, 757, 758
As one collaborative dementia care example, the
Alzheimer’s and Dementia Care (ADC) Program is a health
systems-based model in which clinical providers with
extensive training in dementia care, known as dementia
care specialists (DCSs), co-manage care with the PCP. DCSs
provide comprehensive care addressing medical, behavioral
and social aspects of dementia through the development of
care plans tailored to the needs and goals of each patient
living with dementia and their caregiver. In this
co-management model, the PCP is responsible for the
patient’s primary care needs but shares responsibility for
the dementia-related aspects of care with the DCS,
including reviewing and providing input on the dementia
care plan. The care plan is then implemented by a team, led
by the DCS, that includes family members, other health
professionals and community-based organizations.
625
This
model was found to reduce nursing home admissions for
participating Medicare beneficiaries. The findings were
$601 per patient per quarter ($2,404 per year) in savings,
while the cost of running the program was $317 per patient
per quarter ($1,268 per year), for a net savings of $284 per
patient per quarter ($1,136 per year) for Medicare.
759
As a second example, the Care Ecosystem — a
collaborative, team-based dementia care program utilizing
telehealth that involved care navigators, advanced practice
nurses, social workers and pharmacists — resulted in
fewer ambulance rides, emergency department visits and
hospitalizations and lower total cost of care compared
with usual care.
760, 761
A non-academic health care delivery
organization which adopted the Care Ecosystem found that
the model can be successfully implemented and integrated
into purely clinical settings.
762
With regard to cost savings,
participation in the Care Ecosystem reduced the total cost
of care by $3,290 from 1 to 6 months post-enrollment
and by $3,027 from 7 to 12 months post-enrollment,
corresponding to a mean monthly cost reduction of $526
across 12 months.
761
An implementation toolkit for the
Care Ecosystem is publicly available at: https://memory.
ucsf.edu/sites/memory.ucsf.edu/files/wysiwyg/
CareEcosystemToolkit.pdf.
763
As further evidence of the cost-saving potential of
collaborative dementia care team models, an
interprofessional memory care clinic called the Healthy
Aging Brain Center was shown to reduce per-person
health care costs by $3,474 over a year for individuals with
memory problems, compared with those whose care was
overseen by a PCP only.
761
More than half of the cost
savings were attributed to lower inpatient hospital costs.
The average annual cost of the program was $618 per
person — indicating a nearly 6-to-1 return on investment.
See a description of the new Guiding an Improved
Dementia Experience (GUIDE) Model in the Caregiving
section on page 57 and later in the Workforce section on
page 69 to learn more about efforts to disseminate
collaborative dementia care widely.
Direct Care Workforce
The largest segment of the workforce that supports people
living with dementia is the direct care workforce.
764
Direct
care workers — who are formally classified as personal
care aides, home health aides and nursing assistants, but
known by a wide range of job titles in the field — assist
older adults and people with disabilities in private homes,
community-based settings such as adult day services and
residential care, skilled nursing homes and other settings
such as hospitals.
765
Across these settings, direct care
workers deliver the majority of day-to-day care to patients,
clients or residents living with Alzheimer’s disease and
other forms of dementia.
Direct care workers provide assistance with ADLs, such
as bathing, eating, toilet care and mobility. In home care
settings, they also support individuals with household chores,
meal preparation, attending appointments and other
instrumental activities of daily living (IADLs). Under the
supervision of licensed nurses or other health care
professionals, home health aides and nursing assistants
also perform certain clinical tasks, such as wound care,
measuring vital signs and medication administration
(depending on the setting and regulatory context).
766, 767
Beyond these distinct tasks, direct care workers play a
broader role in promoting nutrition, exercise, functional
ability, social engagement and emotional well-being for
those living with dementia. With training in active listening,
empathic response and other relevant skills, direct care
workers can reduce social isolation, provide emotional
support and, with additional training, help administer
nonpharmacological treatments — such as music and pet
therapy and person-centered bathing — to prevent or
reduce distress associated with dementia.
768-771
Direct care workers also support quality outcomes and cost
savings. Direct care workers providing in-home care enable
individuals to continue living at home and help prevent or
delay nursing home placement.
772
Across settings, they also
provide care to individuals returning from a hospital stay
and can help reduce the risk of readmission, as well as assist
with end-of-life care transitions.
773-776
Thanks to their daily
caregiving role, direct care workers are well-placed to
observe and report changes of status to clinical colleagues,
thereby helping to reduce the risk of emergency
department visits, avoidable hospitalizations and other
adverse outcomes that are disproportionately high among
people living with dementia.
777, 778
Research suggests that
with enhanced dementia-specific training, direct care
workers may also play a role in reducing inappropriate
antipsychotic prescribing for individuals living with dementia
in nursing homes.
779
Between 2012 and 2022, the number of direct care workers
increased from 3.2 million to 4.8 million due to growing
demand for long-term care.
765
Looking ahead, just over
1 million additional direct care workers will be needed
between 2021 and 2031 — more new workers than in any
other single occupation in the United States.
765
This job
growth is occurring primarily among personal care aides and
home health aides, reflecting the overwhelming preference
for “aging in place” and public policies that have expanded
access to home and community-based services.
780
This projected growth in the direct care workforce is seen
across the country. As shown in Table 14, page 63, double-
digit percentage increases in the number of home health
and personal care aides will be needed between 2020 and
2030 to meet demand in every state except Maine. (Unlike
the national workforce projections, updated state-specific
projections will not be available until mid-2024.) Twenty-one
states are expected to see a 30% to 40% increase in the size
of this workforce, while in two states (Arizona and Nevada)
the workforce is expected to increase more than 50%.
Although sizable, these employment projections fall short of
65Workforce
true workforce demand, as they do not account for the
additional workers who will be needed through the
“gray market,” meaning private-pay, usually unreported
employment arrangements. One study using a nationally
representative sample of adults found that nearly a third of
people who arrange paid care for an aging adult or person
living with dementia rely on the gray market (rather than
a home care agency or other formal care provider).
781
Although more direct care workers will be needed in the
years ahead, the long-term care field is already struggling
to fill existing direct care positions. Turnover rates are high
in this workforce — with an estimated median rate of
77% annually for direct care workers providing home
care
782
and 99% for nursing assistants in nursing homes
783
— and recruitment and retention are long-standing
challenges.
784-786
In turn, instability in the workforce and
understaffing across care settings can lead to stress, injury
and burnout among direct care workers, thereby further
contributing to turnover while also compromising care
access and quality.
787, 788
Workforce challenges are driven by persistently low
compensation and poor job conditions for direct care
workers, which are in turn underpinned by structural racial
and gender inequities (that marginalize this workforce
composed predominantly of women and people of color),
765
as well as ageism and disablism (toward the individuals
receiving care and, by extension, those providing it).
789
According to the most recent national data available, the
median wage for direct care workers is just $15.43 per
hour and, due to low wages and the high prevalence of
part-time positions, median annual earnings are less than
$24,000.
765
Research shows that, despite their complex
and critical role in supporting the health and well-being of
older adults and people with disabilities, direct care workers
earn a lower median wage than workers in other “entry-
level” occupations with similar education and training
requirements, such as janitors, retail salespersons and
customer service representatives.
790
Direct care workers also receive limited training and
professional development opportunities, another indicator
of poor job conditions. Nursing assistants in nursing homes
and home health aides employed by Medicare-certified
home health agencies are required by federal regulations
to complete at least 75 hours of entry-level training and
12 hours of annual continuing education (although many
states have set higher training requirements).
786
Care for
individuals with cognitive impairment is among the requisite
training topics for nursing assistants, but not for home
health aides. In contrast, training requirements for other
direct care workers vary by state and setting. With regards
to dementia-specific training, a 2015 review found that
only 13 states had established dementia care training
requirements for direct care workers who provide in-home

66 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Impact of COVID-19 on the Workforce
The COVID-19 pandemic has had a significant
and enduring impact on the health care workforce
and especially on the dementia care workforce,
given the disproportionately high infection and
death rates due to COVID-19 among people living
with Alzheimer’s disease and other dementias.
816
At the onset of the pandemic, the number of people
employed in health care fell from 16.2 to 14.9 million, an
unprecedented decrease of more than 8%.
817, 818
As of
October 2023, the number of people employed in the
health sector was 3.9% higher than in February 2020,
compared with 2.9% higher in all other sectors — but health
sector employment still remained below expected levels
(i.e., there were nearly 482,000 fewer jobs in October 2023
than would have been expected without the pandemic).
817, 818
Employment levels in nursing homes and community care
settings — where a significant proportion of dementia care
takes place — are still far below pre-pandemic levels and
direct care workforce shortages remain acute.
817
As of
October 2023, employment was nearly 10% lower in nursing
homes and 1.3% lower in assisted living and continuing care
retirement communities than in February 2020.
817, 818
Some of the initial job loss in health care was caused by
changes in service delivery and utilization. Elective
procedures were canceled, routine and preventive care visits
were postponed, and admissions into congregate care
settings such as nursing homes were avoided if possible.
Health care workers also had to leave their jobs to safeguard
their own or their families’ health because of illness, or for
caregiving or other reasons. As one startling example of how
COVID-19 directly impacted health care workers, more than
1.7 million COVID-19 cases had been confirmed among
nursing home staff as of October 2023 and over 3,000
nursing home staff had died from the disease.
819
Working during the COVID-19 pandemic has also taken
a significant emotional and psychological toll on the
health care workforce.
820-822
As stated by the U.S.
Surgeon General, “COVID-19 has been a fully and uniquely
traumatic experience for the health workforce, and for their
families.”
823
A systematic review of research published in the
first year of the pandemic found evidence for increased
levels of burnout, emotional exhaustion, depersonalization
and compassion fatigue among health care workers, with
nurses, women and those working directly with COVID-19
patients most impacted.
824
One survey of nearly 21,000 U.S.
health care workers in 2020 found that stress related to
workload and mental health was highest among nursing
assistants, medical assistants and social workers versus other
occupational groups, workers in inpatient versus outpatient
settings, women versus men, and Black and Latinx workers
versus White workers.
825
Researchers are now assessing
these outcomes over time; one example is the longitudinal
COVID-19 Study of Healthcare and Support Personnel
(CHAMPS), which aims to document the effects of the
pandemic on the long-term physical and mental health of
the health care workforce.
826
Participants were recruited for
the initial CHAMPS study in 2020–2021 and, if they
consented to be recontacted, were surveyed at six months’
follow-up and will be surveyed annually thereafter.
The workforce employed by home and community-based
services who assist community-dwelling older adults,
including people living with dementia and their caregivers,
such as those in adult day programs or who deliver
congregate meals, developed new strategies to provide
services and programming to maintain safety, improve
socialization and reduce isolation and loneliness during
lockdown periods. These strategies included providing
home-delivered groceries or grab-and-go meals; facilitating
virtual and remote socialization activities; engaging in
vaccine education and distribution; and deploying
technology, digital literacy and device support,
827-829
with
many of these remote and virtual activities still ongoing.
For the dementia care workforce, the trauma of caring
for those most vulnerable to COVID-19 (and related
challenges, such as social isolation) has likely been
significant.
830
Given the preexisting shortages among
different segments of this workforce, the longer-term
impact of this crisis on workforce recruitment and retention
— as well as on individual health and well-being — must be
closely monitored.
826
care. According to the same review, 44 states and the
District of Columbia had set dementia care training standards
for assisted living staff, but those regulations only pertained to
special dementia care facilities or units in 14 of those states.
791
Inadequate training for direct care workers perpetuates their
mischaracterization as “low-skill” workers, fails to prepare
them for the complexity and challenges of their role,
undermines job satisfaction and retention, and directly
impacts the provision of dementia care.
Direct care is also physically and emotionally demanding
work, which is not well-reflected in the training standards
or compensation for this workforce. As one indicator,
occupational injury data from the Bureau of Labor
Statistics show that nursing assistants in nursing homes
were nearly eight times more likely than U.S. workers
overall to experience workplace injuries in 2020 (the most
recent available year of occupation-specific data on injuries
in nursing homes).
765
These data reflect the impact of the
COVID-19 pandemic on this workforce — as COVID-19
was classified as a “workplace injury”
792
— as well as
long-standing occupational risks.
793
Comparable
occupational injury data are not available for direct care
workers in home and community-based settings due to
reporting limitations, but these workers are also exposed
to a range of occupational risks, including unsafe physical
environments, infection hazards, interpersonal violence
and more.
794
Dementia-Friendly Initiatives and the
Community-Based Workforce
The term “dementia-friendly” has become increasingly
common to describe initiatives to make local communities,
environments and health and social systems more
supportive of people living with dementia and their
caregivers.
795
Work on dementia-friendly communities
began in Japan as early as 2004, with a nationwide campaign
to better understand dementia and build supportive
community networks, which inspired growth of the
movement worldwide.
796
In the U.S., the Dementia-Friendly
America (DFA) initiative launched in 2015 and was described
as a first-of-its-kind national effort that was announced at
the White House Conference on Aging.
797
DFA was built on
the leadership of ACT on Alzheimer’s, a community-led
initiative in Minnesota that began in 2013.
798
There are other
ever-evolving dementia-friendly efforts as well that
encompass a range of settings and contexts, including
dementia-friendly care for people living in hospitals;
799-801
dementia-friendly design for nursing homes, senior centers,
and similar settings;
802-804
and dementia-friendly
neighborhood efforts to improve quality of life for local
residents.
805, 806
Research is still needed on the effectiveness
of these various dementia-friendly efforts.
67Workforce
Looking to the Future
In 2020, the American Public Health Association (APHA)
identified “strengthening the dementia care workforce” as
a public health priority.
831
“Continued failure to strengthen
the dementia care workforce,” according to the APHA, “will
increasingly limit the ability of people living with dementia to
access quality services and supports, adding to health, social
and economic burdens for individuals, families and society.”
This section outlines four emergent areas that will
strengthen the dementia care workforce into the future.
Health Care Workforce Development
First, the health care workforce must expand overall to
meet the needs of the rapidly growing population of older
adults, who are at the highest risk of developing Alzheimer’s
disease and other dementias (see Prevalence section,
page 21).
832
More PCPs, geriatricians, physician assistants,
nurse practitioners, psychologists, therapists, social workers,
direct care workers, other health care workers and
community-based workers who are specifically trained in
caring for people living with dementia will be critically
needed in the years ahead.
One important effort to build the health care workforce
is the Geriatrics Workforce Enhancement Program
(GWEP) funded by the Health Resources and Services
Administration, which comprises a network of 48 GWEPs
across most U.S. states and two territories.
833
The goals of
this program are to educate and train the health care
workforce to provide value-based care for older adults in
integrated geriatrics and primary care models and to deliver
community-based programs that improve health outcomes
for older adults. One particular goal for the GWEPs is to
provide dementia training to a broad range of health care
professionals, educators, individuals and families.
To support people living with dementia in their homes and
communities, as well as their family caregivers, greater
dementia-related knowledge, skills and competencies are
needed in the workforce beyond health care. For instance,
dementia gatekeeper programs have had some success
identifying and supporting people with dementia by training
postal workers, bank tellers, ministers and other personnel
to identify signs of cognitive impairment in older adults and
provide appropriate direction to services.
807
Additional
workforces that play a role in creating dementia-friendly
environments include librarians who provide supportive
services and programming;
808
architects and others who
design floor plans, landscapes, soundscapes and sonic
environments;
802, 809
adult protective service workers who
handle elder abuse cases;
810, 811
police officers and law
enforcement agencies that interact with the public;
812, 813
and hairdressers,
814
bus drivers and building superintendents
among others.
815
68 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Additionally, recognizing the need for expanded training for
professionals who serve older adults, the Substance Abuse
and Mental Health Services Administration (SAMHSA) has
funded a Center of Excellence for Behavioral Health
Disparities in Aging and a Center of Excellence for Building
Capacity in Nursing Facilities to Care for Residents with
Behavioral Health Conditions.
In 2023, the National Institute on Aging funded the National
Dementia Workforce Study (NDWS) under the leadership of
a team of experts in survey research, health workforce
research, and clinical care of people living with dementia.
The NDWS will build a data infrastructure to inform efforts
to strengthen the workforce of clinicians and other care
providers required by the growing population of people
living with dementia in the United States.
834
Dementia Training and Specialization
Targeted dementia training and specialization among PCPs
and across the health care workforce is also needed,
835, 836
including training to address PCPs’ lack of confidence in
diagnosing dementia and communicating that diagnosis,
as discussed earlier. Training in cultural and linguistic
competency is also needed to help the dementia care
workforce better support individuals from diverse
populations, including individuals from various racial and
ethnic and sexual and gender minority groups. Moreover,
language-concordant and culturally tailored resources and
referrals are important for overcoming misunderstandings,
biases, misdiagnoses and related disparities experienced by
people of color and other individuals in minority populations
who are living with dementia and by their families.
837-842
One successful training model is the Alzheimer’s and
Dementia Care ECHO® Program, which pairs PCPs with
multidisciplinary specialist teams through telementoring to
develop their knowledge and confidence in dementia care.
According to an evaluation of the program, which was
launched in 2018 by the Alzheimer’s Association, 94% of
surveyed providers participating in the program reported
making changes in their delivery of dementia care due to
the program and 87% reported higher job satisfaction.
843
Another burgeoning program is Dementia Care Aware,
a state-wide program in California that equips PCPs with
information and tools to successfully administer cognitive
health assessments and determine appropriate next steps
for patients.
844
Once they have completed the online
training, PCPs can receive $29 for providing an annual
screening for cognitive impairment (current procedural
terminology code 1494F) for California Medicaid patients
who are fee-for-service and do not have Medicare.
845
If the initial screening leads to concerns regarding
cognitive impairment, providers can receive $246 for
conducting a cognitive assessment and providing care
plan services (current procedural terminology code 99483)
for California Medicaid patients (Medicare reimburses
approximately $266).
716, 845, 846
The Gerontological Society of America’s Kickstart, Assess,
Evaluate, Refer (KAER) model provides another example of
how to expand the workforce to better detect and manage
dementia.
847
Among other strategies, this model suggests
that non-clinical office staff are well-positioned to
participate in the primary care team’s efforts to detect
cognitive impairment. Receptionists or schedulers, for
example, can take note when patients miss their
appointments, show up at the wrong time, defer to family
members while completing paperwork or answering
questions, or have difficulty following care plans.
Nurse practitioners, physician assistants and other care
providers can also play a greater role in dementia
care delivery, particularly for rural and underserved
communities.
743, 848
With training, support and recognition,
direct care workers can also provide more tailored care for
people living with dementia, for example, by implementing
non-pharmacological interventions to mitigate distress;
observing and recording changes to clinical team members;
and educating and supporting family members.
849
Furthermore, as new therapies for Alzheimer’s and other
dementias develop, the composition and size of the dementia
care workforce must continue to evolve. For example, the
U.S. Food and Drug Administration recently approved two
drugs for the treatment of Alzheimer’s that are delivered
through intravenous infusion and require careful
monitoring of patients for a serious potential side effect
called amyloid-related imaging abnormalities (ARIA, for
more information, see the Overview, page 4). Ensuring the
health of individuals while they receive these drugs requires
an expanded workforce including infusion nurses, radiologists
and radiology technicians with special training in recognizing
ARIA, and specialists with expertise in managing ARIA if it
occurs. Neuropsychologists and other health professionals
are also needed to evaluate whether individuals are
benefiting from the treatments, as those who do not
experience improvements in cognitive skills and the ability to
perform ADLs may be advised to discontinue treatment.
Many people living with dementia move to a nursing home
or other group home setting because there are inadequate
community-based services and supports in their local
area.
850
To create dementia-friendly communities that
support people to age in place, it will be important to also
bolster the dementia knowledge and skills of the broader
community-based workforce — such as postal workers,
bank tellers, ministers, church leaders, librarians, police
officers, building superintendents, bus drivers and hair
dressers.
483, 624, 807, 808, 812-815
For instance, financial advisors
69
are on the frontline with their clients. Research conducted
by Bank of America found that Alzheimer’s dementia was the
most feared condition in later life among the bank’s clients
across all ages and genders. Given this insight, Bank of
America created training programs for their financial
advisors on both Alzheimer’s dementia and caregiving.
851
Payment Models to Support the Dementia Care Workforce
Alternative payment models may be needed to scale-up the
delivery of collaborative, comprehensive and innovative
dementia care.
758, 852, 853
One development in this area is that
since 2017 Medicare reimburses physicians, nurse
practitioners, physician assistants and nurse specialists for
health care visits that result in a comprehensive dementia
care plan. Reimbursement requires cognition-focused
evaluation, identification of caregiver needs, and
development, revision or review of an advance care plan.
Early uptake of this benefit has been limited; a study using a
20% nationwide random sample of eligible fee-for-service
Medicare beneficiaries’ claims data found that only 0.65%
had received this benefit in the first two years.
854
The
authors of this study concluded that providers may be
insufficiently aware of these billing codes, especially in
smaller practices and rural areas, and/or may be billing for
similar services under different codes. In the future,
providers could be better informed about these codes, and
the codes could be revised to include other professionals
such as social workers and psychologists as billing entities.
Another development in the area of payment models is
the nationwide voluntary GUIDE (Guiding an Improved
Dementia Experience) Model, announced by CMS in
2023.
692, 855
Through the GUIDE Model, participating
organizations will offer dementia care programs that provide
ongoing, longitudinal care and support for dually eligible
Medicare and Medicaid people living with dementia and their
caregivers, through an interdisciplinary team. Each team
must include a knowledgeable and skilled care navigator to
help people living with dementia and their caregivers access
clinical and non-clinical services and supports, such as
person-centered assessments and care plans, care
coordination, caregiver training and education, meals and
transportation through community-based organizations,
and 24/7 access to a support line. CMS will test an
alternative payment for participating organizations, who
must be Medicare Part B enrolled providers/suppliers and
eligible to bill for Medicare Physician Fee Schedule services.
To address racial health disparities and inequities in dementia
care, CMS will actively seek out safety-net organizations
that provide care to underserved communities to participate
in the GUIDE Model and will provide financial and technical
supports to ensure they can develop their infrastructure,
improve their workforce and care delivery capabilities, and
participate successfully.
855
Financing and other public policy reforms are also needed
to strengthen and stabilize the direct care workforce. On
a hopeful note, the federal government and states are taking
unprecedented action to improve job quality and bolster
this workforce, particularly through Medicaid, including by
overhauling training and credentialing systems, designing
new career development opportunities, implementing
reimbursement rate increases tied to increased
compensation, developing new recruitment campaigns and
pipeline programs and more.
856, 857
The challenge will be to
sustain these investments into the future, as the need for
direct care services continues to escalate.
Technology to Augment Dementia Care Delivery
Major advances in technology are optimizing the time and
effectiveness of the dementia care workforce. As one
example, e-learning programs can greatly increase access
to dementia care training, although evidence suggests
that the effectiveness of such programs relies on the
relevance of the content and the inclusion of interactive
learning strategies.
858
Technology is also helping to improve access to care for
people living with dementia, especially for those in rural
areas and those with mobility limitations.
859
A randomized
clinical trial of more than 1,500 individuals across urban and
rural areas in California, Nebraska and Iowa to determine
whether telephone- and internet-based delivery of the Care
Ecosystem (a program described on page 64) was effective
in improving outcomes found that the intervention resulted
in better quality of life, reduced emergency department
visits and decreased caregiver depression and burden.
760
A systematic review of telehealth for dementia care,
including routine care, cognitive assessment and
rehabilitation, found that telehealth delivered results similar
to those of in-person services.
860
More research is needed
to identify the strengths and weaknesses of telehealth and
how it can be utilized appropriately in the diagnosis and
treatment of individuals living with dementia, as well as in
supporting their caregivers.
Assistive, therapeutic and remote monitoring technologies,
which range from smart home devices to automated
medication prompts to robotic animals and devices that
support personalized activities and much more, can be used
to augment the role of the dementia care workforce.
861
As with telehealth, more research is needed to understand
the efficacy of these myriad different technologies and to
address concerns and unintended consequences related to
privacy, autonomy and interpersonal interactions. As the
2020 report of The Lancet Commission on dementia
prevention, intervention, and care concluded, “technology
is not a replacement for human contact.”
107
Workforce

Use and Costs of Health Care,
Long-Term Care and Hospice
In 2024, health and long-term
care costs for people living with
Alzheimer’s and other dementias
are projected to reach $360 billion.

The costs of health care and long-term care for
individuals with Alzheimer’s or other dementias are
substantial, and dementia is one of the costliest
conditions to society.
862
Total Cost of Health Care and Long-Term Care
Table 15 reports the average annual per-person
payments for health care and long-term care services for
fee-for-service (i.e., traditional) Medicare beneficiaries
age 65 and older with and without Alzheimer’s or other
dementias based on data from the 2018 Medicare Current
Beneficiary Survey.
A13
Unless otherwise noted, cost and
health care utilization statistics for Medicare beneficiaries
are for fee-for-service Medicare and do not represent
those enrolled in Medicare Advantage.
A14
Total per-person
health care and long-term care payments in 2023 dollars
from all sources for Medicare beneficiaries with
Alzheimer’s or other dementias were nearly three times as
great as payments for other Medicare beneficiaries in the
same age group ($43,644 per person for those with
dementia compared with $14,660 per person for those
without dementia).
A15, 863
Despite having Medicare and other sources of financial
assistance, individuals with Alzheimer’s or other
dementias and their family members still incur high
out-of-pocket costs. These costs are for Medicare
deductibles, copayments and coinsurance; other health
insurance premiums, deductibles, copayments and
coinsurance; and services not covered by Medicare,
Medicaid or other sources of support. On average,
Medicare beneficiaries age 65 and older with Alzheimer’s
or other dementias paid $10,289 out of pocket annually
for health care and long-term care services not covered
by other sources (Table 15).
863
One group of researchers
found that out-of-pocket and informal caregiving costs
for a family member with dementia total $203,117 in
2016 dollars ($240,046 in 2023 dollars) in the last seven
years of life, compared with $102,955 in 2016 dollars
($121,674 in 2023 dollars) for those without dementia.
582
However, informal caregiving costs in the last seven years
of life were considerably higher for households with a
family member with dementia living in the community
compared with households with a family member with
dementia living in a nursing home ($231,730 versus
$165,910 in 2016 dollars [$273,862 versus $196,075
in 2023 dollars]), due to Medicaid covering the cost of
nursing home care for many individuals.
582
Total payments in 2024 (in 2024 dollars) for all
individuals with Alzheimer’s or other dementias
are estimated at $360 billion (Figure 13), not
including the value of informal caregiving
that is described in the Caregiving section.
Medicare and Medicaid are expected to cover
$231 billion, or 64%, of the total health care
and long-term care payments for people with
Alzheimer’s or other dementias. Out-of-pocket
spending is expected to be $91 billion, or
25% of total payments.
A11
For the remainder
of this section, costs are reported in 2023
dollars unless otherwise indicated.
A12
With
the exception of the section, “The COVID-19
Pandemic and Health Care Utilization and
Costs,” data reported in this section reflect
patterns of use before the pandemic. It is
unclear at this point what long-term effect the
pandemic will have on these patterns.
*Data are in 2024 dollars. “Other” payment sources include private
insurance, health maintenance organizations, other managed care
organizations and uncompensated care. The sum of individual dollar
amounts does not equal the total cost due to rounding. Before
rounding, Medicare and Medicaid costs totaled $231 billion.
Created from data from the Lewin Model.
A11
Medicare
$164 B, 45%
Total cost:
$360 Billion (B)
Medicaid
$68 B, 19%
Out of pocket
$91 B, 25%
Other
$38 B, 11%
Distribution of Aggregate Costs of Care by Payment
Source for Americans Age 65 and Older with Alzheimer’s
or Other Dementias, 2024*
71Use and Costs of Health Care, Long-Term Care and Hospice
Figure
13

Researchers have evaluated the additional or
“incremental” health care, residential long-term care
and family caregiving costs of dementia (that is, the
costs specifically attributed to dementia when
comparing people with and without dementia who have
the same coexisting medical conditions and demographic
characteristics).
431, 862, 864, 865
These studies have used
different time horizons, ranging from lifetime costs
(i.e., costs between the time of diagnosis and death) to
annual costs. The lifetime total cost of care, including
out-of-pocket expenses, Medicare and Medicaid
expenditures, and informal caregiving is estimated at
$321,780 per person with Alzheimer’s dementia in
2015 dollars ($394,683 in 2023 dollars), more than
twice the estimated lifetime cost for individuals without
Alzheimer’s dementia).
430
Another group of researchers
found that lifetime total costs were three times higher
for women compared with men with Alzheimer’s
dementia, due to women having a longer duration of
illness and spending more time in a nursing home.
866
Annual incremental health care and nursing home costs
for individuals with dementia (that is, the additional costs
compared with those for individuals without dementia)
are estimated at $28,501 per person per year in 2010
dollars ($40,209 in 2023 dollars).
A16, 862
The majority of
incremental costs have been attributed to informal care
and out-of-pocket costs, rather than medical care and
nursing home costs paid by Medicare or Medicaid.
430, 866, 867
The incremental five-year cost of care for dementia paid
by Medicare has been estimated at nearly $16,000 per
person in 2017 dollars ($22,573 in 2023 dollars), with
nearly half of these costs incurred in the year after
diagnosis and 87% concentrated in the two years after
diagnosis.
867, 868
However, these estimates include costs
for individuals who died during the five-year time period,
and the incremental costs for individuals who survive at
least five years after diagnosis are even higher.
Several groups of researchers have specifically examined
out-of-pocket costs and found that individuals with
Alzheimer’s or other dementias and their families incur
substantially higher out-of-pocket costs compared with
individuals without Alzheimer’s. Although incremental
Medicare expenditures peak in the year after diagnosis and
decrease in the subsequent four years, out-of-pocket costs
have been shown to increase over time, from $3,104 in
the first two years after diagnosis to $3,730 in years three
to four after diagnosis, to $3,934 in years seven to eight
after diagnosis (in 2017 dollars; $3,579, $4,300 and
$4,535 in 2023 dollars).
869
Higher out-of-pocket costs for
Alzheimer’s and other dementias have been attributed to
nursing home care, home health care and prescription drug
payments.
870, 871
Furthermore, individuals with Alzheimer’s
dementia spend 12% of their annual income on out-of-
pocket health care services on average, excluding nursing
home and informal care, compared with 7% for individuals
without Alzheimer’s dementia.
871
Another perspective to examine incremental costs for
individuals with Alzheimer’s and other dementias is
through end-of-life costs. A recent systematic review of
end-of-life costs for individuals with dementia reported
that costs were especially high during the last month of
life, even compared with monthly costs over the last year
of life.
872
Researchers comparing end-of-life costs in the
last five years of life for individuals with and without
dementia found that the total cost was $287,038 per
person for individuals with dementia in 2010 dollars and
$183,001 per person for individuals without dementia
($404,949 and $258,175, respectively, in 2023 dollars),
a difference of 57%.
873
Out-of-pocket costs represent
a substantially larger proportion of total wealth for
those with dementia than for people without dementia
(32% versus 11%).
72 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Payment
Source
Beneficiaries
with Alzheimer’s
or Other
Dementias
Beneficiaries
without
Alzheimer’s or
Other Dementias
Medicare $21,973 $7,918
Medicaid 6,771 305
Uncompensated 192 240
Health maintenance
organization 1,952 2,292
Private insurance 1,534 958
Other payer 933 419
Out of pocket 10,289 2,529
All sources 43,644 14,660
*Payments for beneficiaries with Alzheimer’s or other dementias
include payments for community-dwelling beneficiaries and
beneficiaries residing in residential care facilities.
Created from unpublished data from the Medicare Current
Beneficiary Survey for 2018.
863
Average Annual Per-Person Payments by Payment Source
for Health Care and Long-Term Care Services, Medicare
Beneficiaries Age 65 and Older, with and without
Alzheimer’s or Other Dementias, in 2023 Dollars*
Table
15

Use and Costs of Health Care Services
Use of Health Care Services
Unadjusted data (that is, data that don’t account for
differences in the characteristics of people with versus
without Alzheimer’s or other dementias) show that people
with Alzheimer’s or other dementias have more than
twice as many hospital stays per year as other older
people.
413
Moreover, the use of health care services by
people with other serious medical conditions is strongly
affected by the presence or absence of dementia. In
particular, people with coronary artery disease, diabetes,
chronic kidney disease, chronic obstructive pulmonary
disease, stroke or cancer who also have Alzheimer’s or
other dementias have higher use and costs of health care
services than people with these medical conditions but
no coexisting dementia.
In addition to having more hospital stays, older people
with Alzheimer’s or other dementias have more skilled
nursing facility stays per year than other older people.
• Hospital. In 2019, there were 518 hospital stays per
1,000 Medicare beneficiaries age 65 and older with
Alzheimer’s or other dementias compared with 234
hospital stays per 1,000 Medicare beneficiaries age 65
and older without these conditions.
413
Overall, 32%
of Medicare beneficiaries with Alzheimer’s or other
dementias have at least one hospital discharge annually
compared with 15% of beneficiaries without these
conditions, with average hospital lengths of stay of
5.1 days versus 4.5 days, respectively.
413
The most
common reasons that people with Alzheimer’s
dementia are hospitalized are not due to the disease
itself, but for other reasons, including syncope
(fainting), fall and trauma (26%), ischemic heart disease
(17%), and gastrointestinal disease (9%),
874
although
the COVID-19 pandemic may have changed the most
common reasons for hospitalization starting in 2020.
A study of inpatient hospitalizations of adults age 60
and older found that those with Alzheimer’s dementia
were at 7% greater risk of dying during the hospital
stay and stayed nearly a day longer than individuals
without Alzheimer’s dementia.
875
Among Medicare
beneficiaries with Alzheimer’s or other dementias,
22% of hospital stays are followed by a readmission
within 30 days.
854
Although not directly comparable,
one study of a random sample of Medicare
beneficiaries from 50 U.S. hospital referral regions
found an overall 30-day readmission rate of 18%.
876
The proportion of hospital stays followed by a
readmission within 30 days remained relatively
constant between 2008 and 2018 for Medicare
beneficiaries with Alzheimer’s and related dementias
(23% in 2008 versus 22% in 2018).
877
• Emergency department. There were nearly 1.8 million
emergency department visits for people with
Alzheimer’s in 2021, representing 1.3% of all
emergency department visits.
878
There are 1,545
emergency department visits per 1,000 Medicare
beneficiaries with Alzheimer’s or other dementias per
year, including emergency department visits that result
in a hospital admission.
877
Although not directly
comparable, there were 640 emergency department
visits per 1,000 Medicare beneficiaries per year based
on a review of utilization patterns of a subset of
Medicare beneficiaries.
876
Emergency department
visits for people with Alzheimer’s or other dementias
increased 22% between 2008 and 2018 (from 1,265
to 1,545 per 1,000 Medicare beneficiaries), exceeding
the increases in emergency department visits for
individuals with cancer, ischemic heart disease and
heart failure among others (Figure 14, page 74).
877
One group of researchers found that individuals
with Alzheimer’s or another dementia seen in the
emergency department are more likely to be admitted
to the hospital or a nursing home from the emergency
department than Medicare beneficiaries without
Alzheimer’s or other dementias.
879
Additionally,
individuals with Alzheimer’s or other dementias are
more likely to have at least one hospitalization, have at
least one subsequent emergency department visit and
be admitted to hospice in the 12 months following the
initial emergency department visit.
• Skilled nursing facility. Skilled nursing facilities provide
direct medical care that is performed or supervised by
registered nurses, such as giving intravenous fluids,
changing dressings, administering tube feedings and
providing around-the-clock personal care services.
880
There are 188 skilled nursing facility stays covered by
Medicare per 1,000 Medicare beneficiaries with
Alzheimer’s or other dementias per year compared
with 40 stays per 1,000 beneficiaries without these
conditions — a rate nearly five times as high.
413
Overall, 19% of Medicare beneficiaries with Alzheimer’s
or other dementias have at least one skilled nursing
facility stay annually compared with 4% of Medicare
beneficiaries without these conditions.
413
73Use and Costs of Health Care, Long-Term Care and Hospice

Costs of Health Care Services
Average per-person payments for health care services
(hospital, physician and other medical provider, nursing
home, skilled nursing facility, hospice and home health
care) and prescription medications were higher for
Medicare beneficiaries with Alzheimer’s or other dementias
than for Medicare beneficiaries without dementia in the
same age group (see Table 16).
A13, 863
Use and Costs of Health Care Services by State
Substantial geographic variation exists in health care
utilization and Medicare payments by individuals with
Alzheimer’s or other dementias (see Table 17, page 77).
Emergency department visits, including visits that result
in a hospital admission, range from 1,154 per 1,000
beneficiaries annually in Nebraska to 1,811 per 1,000
beneficiaries annually in West Virginia, and the
percentage of hospital stays followed by hospital
readmission within 30 days ranges from 16% in Hawaii to
25.8% in Nevada. Medicare spending per capita ranged
from $21,711 in North Dakota to $44,310 in California.
877
Use and Costs of Health Care Services Across the
Alzheimer’s Disease Continuum
Health care costs increase with the presence of
dementia. In a population-based study of adults age 70
to 89, annual health care costs were significantly higher
for individuals with dementia than for those with either
mild cognitive impairment (MCI) or without cognitive
impairment.
882
Annual health care costs for individuals
with MCI were not significantly different, however, from
costs for individuals without cognitive impairment.
Several groups of researchers have found that both
health care and prescription drug spending are
significantly higher for people diagnosed with Alzheimer’s
or other dementias in the year prior to diagnosis,
883-885
although the sources of increased spending differed
across these studies. In one study, the largest differences
in spending were due to inpatient and post-acute
care,
884
while in another study the differences in
spending were primarily due to outpatient care, home
care and medical day services, with only a small
difference in inpatient care costs.
885
*Includes Medicare beneficiaries with a claims-based diagnosis of each chronic condition. Beneficiaries may have more than one chronic condition.
Created from data from the U.S. Centers for Medicare & Medicaid Services.
877
Percentage
30
25
20
15
10
5
0
-5
-10
6%
9% 9% 9%
14%
23%
26%
28%
-9%
Percentage Changes in Emergency Department Visits per 1,000 Fee-for-Service
Medicare Beneficiaries with Selected Health Conditions Between 2008 and 2018*
Chronic
kidney
disease
Health
condition
Diabetes Cancer StrokeIschemic
heart
disease
HypertensionCOPD Heart
failure
Alzheimer’s
and other
dementias
74 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Figure
14

Three groups of researchers have found that spending in
the year after diagnosis was substantially higher than
spending for individuals who had similar characteristics but
did not have Alzheimer’s or dementia, by amounts ranging
from $7,264 in 2017 dollars ($8,374 in 2023 dollars)
867
based on individuals with fee-for-service (i.e., traditional)
Medicare coverage, to $17,852 in additional costs in 2014
dollars ($22,473 in 2023 dollars)
884
based on another
group of individuals with fee-for-service Medicare. One
group of researchers, however, did not find a significant
difference in health care spending in the two years
after diagnosis.
886
Researchers have found that health care costs remain
higher beyond the year after diagnosis. One group of
researchers also found the incremental costs remained
higher in the second year after diagnosis ($7,327 in
additional costs in 2014 dollars [$9,224 in 2023 dollars]).
884
Another research team found that health care costs
remained higher in the second through fourth years after a
dementia diagnosis but were not significantly different
from costs for individuals without the diagnosis in the fifth
year after diagnosis.
867
Incremental costs decreased over
time, from $4,241 in 2014 dollars ($4,889 in 2023 dollars)
in year two to $1,302 ($1,501 in 2023 dollars) in year four,
although costs increase dramatically in the last year and last
month of life.
854
Researchers have also found a similar
increase in health care costs in the year before and two
years after a diagnosis of MCI, although the additional costs
were lower than costs for Alzheimer’s.
884
One possible
explanation for the spike in health care costs in the year
immediately before and the year immediately after
diagnosis of Alzheimer’s or another dementia relates to
delays in timely diagnosis. One group of researchers found
that individuals with cognitive decline who sought care
from a specialist (that is, a neurologist, psychiatrist or
geriatrician) had a shorter time to diagnosis of Alzheimer’s
disease.
887
Additionally, individuals diagnosed with cognitive
impairment by a specialist had lower Medicare costs in the
year after receiving a diagnosis of Alzheimer’s dementia
than those diagnosed by a non-specialist.
Impact of Alzheimer’s and Other Dementias on
the Use and Costs of Health Care in People with
Coexisting Medical Conditions
Nearly 9 out of 10 Medicare beneficiaries with Alzheimer’s
disease or other dementias have at least one other chronic
condition.
413
Additionally, they are more likely than those
without dementia to have other chronic conditions.
413
Overall, 2.7 times more Medicare beneficiaries with
Alzheimer’s or other dementias have four or more chronic
conditions (excluding Alzheimer’s disease and other
dementias) than Medicare beneficiaries without dementia.
413
Table 18, page 78, reports the percentage of people with
Alzheimer’s or other dementias who had certain coexisting
medical conditions. In 2019, 46% of Medicare beneficiaries
age 65 and older with dementia also had coronary artery
disease, 46% had chronic kidney disease, 37% had diabetes,
34% had congestive heart failure and 20% had chronic
obstructive pulmonary disease.
413
Medicare beneficiaries who have Alzheimer’s or other
dementias and a coexisting medical condition have higher
average per-person payments for most health care
services than Medicare beneficiaries with the same medical
condition but without dementia. Table 19
A13
, page 79,
shows the average per-person Medicare payments for
seven specific medical conditions among beneficiaries who
have Alzheimer’s or other dementias and beneficiaries who
do not have Alzheimer’s or another dementia.
A13, 413
Medicare beneficiaries with Alzheimer’s or other dementias
have higher average per-person payments in all categories
except physician care. Additionally, one group of
researchers found that individuals with dementia and
behavioral disturbances, such as agitation, had more
psychiatric comorbidities than individuals with dementia
but without behavioral disturbances.
888
This group of
researchers also found that larger proportions of
individuals with dementia and behavioral disturbances used
75Use and Costs of Health Care, Long-Term Care and Hospice
*“Medical provider” includes physician, other provider and laboratory
services, and medical equipment and supplies.
†
Information on payments for prescription medications is only
available for people who were living in the community, that is, not
in a nursing home or an assisted living residence.
Created from unpublished data from the Medicare Current
Beneficiary Survey for 2018.
A13, 863
Payment
Source
Beneficiaries
with Alzheimer’s
or Other
Dementias
Beneficiaries
without
Alzheimer’s or
Other Dementias
Inpatient hospital $7,580 $2,836
Outpatient events 2,867 2,256
Medical provider* 5,956 3,844
Skilled nursing facility 3,890 392
Nursing home 14,347 555
Hospice 2,321 136
Home health care 1,857 274
Prescription medications
†
5,016 3,383
Average Annual per-Person Payments by Type of
Service for Health Care and Long-Term Care Services,
Medicare Beneficiaries Age 65 and Older, with and
without Alzheimer’s or Other Dementias, in 2023 Dollars
Table
16

76 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
medications including antihypertensives, dementia
treatments, antipsychotics, antidepressants, antiepileptics
and hypnotics compared with individuals with dementia
but without behavioral disturbances.
Use and Costs of Long-Term Care Services
Long-term care services include home- and community-
based services and services delivered in assisted living
residences and nursing homes. An estimated 65% of older
adults with Alzheimer’s or other dementias live in the
community, compared with 98% of older adults without
Alzheimer’s or other dementias.
863
Of those with
dementia who live in the community, 74% live with
someone and the remaining 26% live alone.
863
As their
disease progresses, people with Alzheimer’s or other
dementias generally receive more care from family
members and other unpaid caregivers. Many people with
dementia also receive paid long-term care services at
home; in adult day centers, assisted living residences or
nursing homes; or in more than one of these settings at
different times during the often long course of the
disease. Medicaid is the only public program that covers
the long nursing home stays that most people with
dementia require in the severe stage of their illnesses.
Use of Long-Term Care Services by Setting
Most people with Alzheimer’s or other dementias who
live at home receive unpaid help from family members
and friends, but some also receive paid home- and
community-based services, such as personal care and
adult day care. People with Alzheimer’s or other
dementias make up a large proportion of all older adults
who receive adult day services and nursing home care.
• Home health services and other home-based services.
Medicare covers home health services, such as
part-time skilled nursing care; skilled therapy services;
home health aide care, such as intermittent help with
bathing, toileting and dressing if needed, with skilled
nursing or therapy services; and medical social services
in the home. Home health agencies provide the
majority of home health care services.
889
Fee-for-
service Medicare does not cover homemaker services,
such as meal preparation, or personal care services,
such as help with bathing, toileting and dressing, if this
is the only care that is needed; however, Medicare
Advantage plans (Medicare Part C) are allowed to offer
these services as supplemental benefits, and 17%
offered in-home support services as a benefit in
2023.
890
Additionally, 16% of Medicare Advantage
plans offered food and produce as a supplemental
benefit, and 8% offered meals beyond a limited basis.
890
These supplemental benefits are more common in
Medicare Advantage Special Needs Plans (i.e., plans
that are designed for Medicare enrollees with specific
needs, such as individuals with a chronic condition,
individuals who are also enrolled in Medicaid, and
institutionalized enrollees), with 41% offering a food
and produce benefit, 31% offering in-home support
services, and 15% offering meals beyond a limited
basis. Although Medicare Advantage Special Needs
Plans can be offered to individuals with specific
chronic conditions, including dementia, only 8% of
Special Needs Plan enrollees are enrolled in a plan for
chronic or disabling conditions, representing less than
1% of all Medicare enrollees.
891, 892
The vast majority
of Special Needs Plan enrollees are individuals also
enrolled in Medicaid (i.e., Dual Eligible Special Needs
Plan enrollees).
Thirty-six percent of individuals using home health
services have Alzheimer’s or other dementias.
893
Of Medicare beneficiaries age 65 and older with
Alzheimer’s or other dementias, 26% have at least one
home health visit paid by Medicare during the year,
compared with 8% of Medicare beneficiaries age 65
and older without Alzheimer’s or other dementias
413
and they use an average of 110 days of home care per
year (including homemaker services and other services
not covered by Medicare) compared with 64 days per
year for individuals age 65 and older without the
disease.
889
Receipt of home health services after
hospital discharge has been shown to increase the
likelihood of remaining in the community for at least
30 days after hospital discharge, with greater benefits
from longer durations of home health care.
894
• Adult day services. The fourth most common chronic
condition in participants using adult day services is
Alzheimer’s disease or other dementias, and 25% of
individuals using adult day services have Alzheimer’s or
other dementias.
893
Fourteen percent of adult day
service centers in the United States specialized in caring
for individuals with Alzheimer’s disease or other
dementias in 2020, up from 10% in 2016.
893, 895
The
percentage of participants with Alzheimer’s or other
dementias was higher in adult day service centers
that provided either low- or moderate-level medical
services than in centers that either provided no medical
services or mainly provided health or medical services.
895
• Residential care facilities. Forty-two percent of
residents in residential care facilities (that is, housing
that includes services to assist with everyday activities,
such as personal care, medication management
and meals), including assisted living facilities, had
Alzheimer’s or other dementias in 2020, up from
34% in 2016.
893, 896
Sixty-one percent of residential
care communities are small (four to 25 beds),
896
and
these facilities have a higher percentage of residents
with Alzheimer’s or other dementias than larger
facilities (51% in facilities with four to 25 beds
compared with 47% in facilities with 26 to 50 beds and

*Based on Medicare utilization for 2018.
†
Based on Medicare utilization for 2022.
Created from data from the U.S. Centers for Medicare & Medicaid Services.
877, 881
State
Number of
ED Visits
per 1,000
Beneficiaries*
Percentage of
Hospital Stays
Followed by
Readmission
within 30 Days*
Per Capita
Medicare
Payments
†
Alabama 1,410.8 21.2 $27,369
Alaska 1,477.6 19.3 29,741
Arizona 1,436.2 20.2 29,111
Arkansas 1,530.4 21.5 27,092
California 1,496.3 23.0 44,310
Colorado 1,424.8 18.6 28,687
Connecticut 1,635.4 22.7 34,958
Delaware 1,577.6 21.5 32,413
District of Columbia 1,741.7 25.6 39,378
Florida 1,551.9 23.0 34,142
Georgia 1,573.2 22.5 30,219
Hawaii 1,248.2 16.0 24,763
Idaho 1,389.2 17.2 25,057
Illinois 1,624.1 23.4 34,637
Indiana 1,514.2 21.3 30,219
Iowa 1,310.7 18.0 22,067
Kansas 1,406.0 19.8 27,260
Kentucky 1,735.5 23.1 29,473
Louisiana 1,709.9 22.1 34,182
Maine 1,665.3 19.7 24,119
Maryland 1,524.1 24.4 37,381
Massachusetts 1,668.4 24.7 36,930
Michigan 1,691.4 24.0 30,432
Minnesota 1,467.1 21.6 27,300
Mississippi 1,714.8 22.1 31,174
Missouri 1,529.6 22.6 28,324
State
Number of
ED Visits
per 1,000
Beneficiaries*
Percentage of
Hospital Stays
Followed by
Readmission
within 30 Days*
Per Capita
Medicare
Payments
†
Montana 1,328.6 16.6 $22,360
Nebraska 1,153.6 18.7 25,345
Nevada 1,711.5 25.8 41,608
New Hampshire 1,493.8 20.4 28,663
New Jersey 1,456.3 22.9 38,795
New Mexico 1,563.7 20.6 27,258
New York 1,461.3 23.7 43,601
North Carolina 1,683.8 21.5 27,591
North Dakota 1,173.3 18.4 21,711
Ohio 1,618.7 22.5 30,661
Oklahoma 1,692.1 21.6 32,584
Oregon 1,628.4 18.7 25,470
Pennsylvania 1,470.5 22.0 31,112
Rhode Island 1,605.6 23.2 30,782
South Carolina 1,558.2 21.7 29,657
South Dakota 1,200.1 18.6 24,776
Tennessee 1,548.6 21.5 28,543
Texas 1,549.1 22.1 37,679
Utah 1,194.3 16.7 26,233
Vermont 1,528.4 19.6 23,329
Virginia 1,621.7 21.6 27,870
Washington 1,479.2 18.6 25,274
West Virginia 1,811.4 24.1 29,240
Wisconsin 1,519.9 19.9 27,335
Wyoming 1,445.9 17.4 25,452
Emergency Department (ED) Visits, Hospital Readmissions and Per Capita Medicare Payments in 2023 Dollars
by Medicare Beneficiaries with Alzheimer’s or Other Dementias
77Use and Costs of Health Care, Long-Term Care and Hospice
Table
17

78 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Created from unpublished data from the National 100% Sample
Medicare Fee-for-Service Beneficiaries for 2019.
413
39% in facilities with more than 50 beds).
897
Fifty-eight
percent of residential care facilities offer activities or
programs for residents with Alzheimer’s or other
dementias.
898
Average aide staff hours per resident day
in residential care communities range from 2.2 hours
per day in facilities with less than 25% of residents
diagnosed with dementia to 2.7 hours per day in
facilities with more than 75% of residents diagnosed
with dementia.
896
• Nursing home care. Overall, 46% of nursing home
residents have Alzheimer’s or other dementias,
893
although the prevalence differs by duration of nursing
home stay. While 36% of short-stay (less than 100
days) nursing home residents have Alzheimer’s or
other dementias, 58% of long-stay (100 days or longer)
residents have these conditions. Twenty-four percent
of Medicare beneficiaries with Alzheimer’s or other
dementias reside in a nursing home, compared with 1%
of Medicare beneficiaries without these conditions.
863
At age 80, approximately 75% of people with
Alzheimer’s dementia live in a nursing home compared
with only 4% of the general population age 80.
415
• Alzheimer’s special care units and dedicated facilities.
An Alzheimer’s special care unit is a dedicated unit, wing
or floor in a nursing home or other residential care
facility that has tailored services for individuals with
Alzheimer’s or other dementias. Thirteen percent of
nursing homes and 21% of assisted living and other
residential care communities have a dementia special
care unit.
893
Less than 1% (0.3%) of nursing homes and
11% of other residential care facilities exclusively
provide care to individuals with dementia.
Long-Term Care Services Provided at Home and
in the Community
Overall, 70% of spending for long-term care services and
supports is covered by public payers, including Medicaid
(43%), Medicare (21%) and other public payers (6%), and 15%
is covered by out-of-pocket payments, including direct
payments and deductibles and copayments for services
covered by another payment source (15%). Private insurance
covers only 9% of long-term services and supports.
899
Nationally, state Medicaid programs are shifting long-term
care services from institutional care to home- and
community-based services as a means to both reduce
unnecessary costs and meet the growing demand for these
services by older adults. The federal and state governments
share the management and funding of Medicaid, and states
differ greatly in the services covered by their Medicaid
programs. In 2020, home- and community-based services
represented the majority (62%) of the $199.4 billion spent
by Medicaid on long-term care services and supports, with
institutional care representing the remaining 38%.
900
However, there is substantial variation across states in
spending on home- and community-based services, ranging
from 32% of total Medicaid long-term care services and
supports in Mississippi to 84% of long-term care services
and supports spending in Oregon, despite evidence
demonstrating that Medicaid spending on these services
reduces costs.
901
Thirty-three percent of Medicaid’s total
expenditures cover expenditures related to long-term care
services and supports.
Between 2010 and 2020, Medicaid spending on
home- and community-based services increased from
48% to 62% of total long-term services and supports
expenditures.
900
Additionally, total spending on home care
for Medicare beneficiaries with Alzheimer’s or other
dementias increased dramatically between 2004 and
2018.
902
Increases in spending may have been due to a
variety of factors, including more people being diagnosed
with Alzheimer’s dementia, more people using home care,
an increase in the number of coexisting medical
conditions, more intensive use of home care services and
an increase in Medicaid coverage for older adults.
902
In
two systematic reviews of the cost-effectiveness of
enhanced home support interventions for individuals with
dementia, researchers found some evidence to support
occupational therapy, home-based exercise, and some
psychological and behavioral treatments as potentially
cost-effective approaches, although research that has
evaluated both the costs and benefits of enhanced home
support interventions is scant.
903, 904
Coexisting Condition Percentage
Coronary artery disease 46
Chronic kidney disease 46
Diabetes 37
Congestive heart failure 34
Chronic obstructive pulmonary disease 20
Stroke 13
Cancer 10
Percentage of Medicare Beneficiaries Age 65 and
Older with Alzheimer's or Other Dementias Who
Have Specified Coexisting Conditions
Table
18

Created from unpublished data from the National 100% Sample Medicare Fee-for-Service Beneficiaries for 2019.
A13,413
Medical Condition by
Alzheimer’s/Dementia
(A/D) Status
Average Per-Person Medicare Payments
Total
Medicare
Payments
Hospital
Care
Physician
Care
Skilled
Nursing
Home Care
Home
Health Care
Hospice
Care
Coronary artery disease
With A/D $28,418 $8,462 $4,815 $4,362 $2,446 $3,799
Without A/D 17,976 6,119 4,718 1,353 938 421
Diabetes
With A/D 28,064 8,478 4,834 4,417 2,353 3,263
Without A/D 15,729 5,213 4,225 1,227 827 291
Congestive heart failure
With A/D 31,433 9,739 5,006 4,928 2,595 4,305
Without A/D 25,415 9,331 5,478 2,385 1,557 798
Chronic kidney disease
With A/D 29,151 8,799 4,791 4,552 2,471 3,857
Without A/D 19,733 6,720 4,918 1,626 1,082 470
Chronic obstructive pulmonary disease
With A/D 31,981 10,055 5,226 5,088 2,622 3,842
Without A/D 22,785 8,086 5,372 1,898 1,298 708
Stroke
With A/D 30,551 9,154 5,069 4,854 2,584 3,753
Without A/D 22,196 7,310 5,226 2,334 1,548 652
Cancer
With A/D 28,352 8,127 5,200 4,076 2,429 3,760
Without A/D 18,330 5,056 5,717 1,032 732 732
Average Annual Per-Person Payments by Type of Service and Coexisting Medical Condition for Medicare
Beneficiaries Age 65 and Older, with and without Alzheimer’s or Other Dementias, in 2023 Dollars
79Use and Costs of Health Care, Long-Term Care and Hospice
Table
19

80 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Transitions Between Care Settings
Individuals with dementia often move between a nursing
facility, hospital and home, rather than remaining solely
in a nursing facility. In a longitudinal study of primary care
patients with dementia, researchers found that individuals
discharged from a nursing facility were nearly equally as
likely to be discharged home (39%) as discharged to a
hospital (44%).
905
Individuals with dementia may also
transition between a nursing facility and hospital or
between a nursing facility, home and hospital, creating
challenges for caregivers and providers to ensure that care
is coordinated across settings. Other researchers have
shown that nursing home residents frequently have
burdensome transitions at the end of life, including
admission to an intensive care unit in the last month of life
and late enrollment in hospice,
906
although the number of
care transitions for nursing home residents with advanced
cognitive impairment varies substantially across geographic
regions of the United States.
907
Costs of Long-Term Care Services
• Home care. The median cost in 2021 for a nonmedical
home health aide was $27 per hour and $5,148 per
month ($28 and $5,341 in 2023 dollars).
908
Nonmedical home care costs increased 5.9% annually
on average between 2017 and 2021. The cost of
homemaker services was $26 per hour and $4,957 per
month ($27 and $5,143 in 2023 dollars) and increased
5.4% annually on average between 2017 and 2021.
• Adult day centers. The median cost of adult day services
was $78 per day in 2021 ($85 in 2023 dollars).
908
The
cost of adult day services increased 2.8% annually on
average between 2017 and 2021.
• Assisted living residences. The median cost for care in
an assisted living residence was $4,500 per month, or
$54,000 per year in 2021 ($4,921 and $59,047 in
2023 dollars).
908
The cost of assisted living increased
4.4% annually on average between 2017 and 2021.
• Nursing homes. The 2021 average cost for a private
room in a nursing home was $297 per day, or
$108,405 per year ($325 and $118,536 in 2023
dollars), and the average cost of a semi-private room
was $260 per day, or $94,900 per year ($284 and
$103,769 in 2023 dollars).
908
The cost of nursing home
care increased 3.3% annually on average for a private
room and 2.9% annually on average for a semi-private
room between 2017 and 2021.
Affordability of Long-Term Care Services
Few individuals with Alzheimer’s or other dementias have
sufficient long-term care insurance or can afford to pay
out of pocket for long-term care services for as long as
the services are needed.
• Medicare beneficiaries with a dementia diagnosis
have lower household incomes on average than
beneficiaries without a dementia diagnosis. In 2018,
23% of community-dwelling Medicare beneficiaries
with a dementia diagnosis had household incomes
below the federal poverty level, and 53% had
household incomes between 100% and 200% of the
federal poverty level, while 15% of those without a
dementia diagnosis lived below the federal poverty
level and 40% had household incomes between 100%
and 200% of the federal poverty level.
909
• Asset data are not available for people with Alzheimer’s
or other dementias specifically, but 50% of Medicare
beneficiaries age 65 and older had total savings of
$83,850 or less in 2019 dollars ($92,188 in 2023
dollars), and 25% had savings of $9,650 or less in 2019
dollars ($10,610 in 2023 dollars). Median savings for
White Medicare beneficiaries were 8.5 times higher
than for Black beneficiaries and more than 15 times
higher than for Hispanic beneficiaries.
910
In a 2022
survey of adults about the affordability of long-term
care, less than one-third (31%) of adults age 65 and
older reported being very confident that they would
have the financial resources to pay for necessary care
as they age.
911
Additionally, of adults age 50 and older,
nearly two-thirds reported feeling anxious about being
able to afford nursing home or assisted living care,
if they should need it. Although individuals from lower
income households were more likely to report feeling
anxious about the affordability of long-term care
(77% with household incomes less than $40,000 reported
being anxious about the affordability of long-term care),
nearly half of individuals from households with incomes
$90,000 or greater also reported being anxious about
the affordability (in 2022 dollars; $41,553 and $93,495,
respectively, in 2023 dollars).
Long-Term Care Insurance
Long-term care insurance typically covers the cost of
care provided in a nursing home, assisted living residence
and Alzheimer’s special care residence, as well as
community-based services such as adult day care and
services provided in the home, including nursing care
and help with personal care.
921

81Use and Costs of Health Care, Long-Term Care and Hospice
Although Medicare covers care in a long-term
care hospital, skilled nursing care in a skilled
nursing home and hospice care, it does not
cover long-term care (i.e., stays more than
90 days) in a nursing home.
912
Results from a 2022 survey about the affordability of
long-term care revealed that 23% of adults believed that
Medicare would cover the cost of nursing home care,
and 28% were not sure who would pay for nursing home
care. Even more concerning, 45% of individuals age
65 and older believed that Medicare would cover the
cost of nursing home care.
911
These findings suggest
that Medicare beneficiaries and caregivers need more
education and information about the types of services
that Medicare covers. In particular, Medicare does not
cover custodial care, that is, care to assist with activities
of daily living, such as dressing and bathing. Most
nursing home care is custodial care, and therefore is
not covered by Medicare.
Medicare does cover post-acute skilled nursing care,
or nursing and therapy care that must be performed
or supervised by medical professionals, such as
registered or licensed nurses.
913
For Medicare to cover
skilled nursing care, the Medicare beneficiary must
have a qualifying hospital stay, a physician must
decide that skilled care is needed, and the medical
condition requiring skilled care must be related to the
hospitalization.
914
Fee-for-service Medicare (Part A)
covers the first 20 days of skilled nursing care with
$0 coinsurance for each benefit period. For the next
80 days of skilled nursing care (days 21-100), the
beneficiary pays $204 per day in coinsurance.
915
A long-term care hospital is an acute care hospital that
specializes in caring for people who stay more than
25 days, on average. A long-term care hospital provides
specialized care, such as respiratory therapy, pain
management and treatment for head trauma.
916
Benefits work in the same way that Medicare covers
other acute care hospitalizations.
The terms “Medicare” and “Medicaid” are also often
confused. Most individuals who are age 65 or older, have
a permanent disability or have end-stage kidney disease
qualify for Medicare Part A, which is also referred to as
hospital insurance.
917
Individuals are eligible to receive
Medicare Part A at no cost if they have worked and paid
Medicare taxes for at least 10 years (i.e., have a sufficient
earnings history) or a spouse, parent or child has a
sufficient earnings history. Medicare Part B (medical
insurance) is a voluntary program that requires enrollees
to pay a monthly premium. Medicare Advantage Plans,
also referred to as Medicare Part C, are becoming more
common, with more than one-half (51%) of Medicare
beneficiaries enrolled in this type of plan in 2023.
918
Advantage Plans are privately offered Medicare plans
that combine Medicare Parts A and B and often also
include prescription drug coverage (Medicare Part D).
919
While Medicare is a federal program, Medicaid is a
joint federal and state program, and benefits vary
state-to-state.
920
Individuals with low incomes and/or
low resources may qualify for Medicaid coverage.
Medicaid covers some services that Medicare either
does not cover or only partially covers, such as
nursing home care and home- and community-based
care. Individuals who are enrolled in both Medicare
and Medicaid are sometimes referred to as being
“dually eligible.”
For more information about Medicare, visit
medicare.gov. For more information about Medicaid,
visit https://www.medicaid.gov/.
Medicare Does Not Cover Long-Term Care in a Nursing Home
82 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Based on data from the National Health Expenditure
Account, it is estimated that private insurance covered
only 9% ($38.5 billion) of the cost of long-term services
and supports in 2019.
899
Industry reports estimate that
between 5.3 and 7.1 million Americans had private
long-term care insurance in 2020-2021.
922, 923
However,
the long-term care insurance market is shrinking, with
only 57,000 new policies sold in 2018, compared with
754,000 in 2002.
924
The average premium for a long-
term care insurance policy was $155 per month in
2021.
923
The private long-term care insurance market
has consolidated since 2000. In 2000, 41% of individuals
with a long-term care policy were insured by one of the
five largest insurers versus 60% in 2020.
913, 922
Cognitive
conditions are the most common final diagnosis for
long-term care insurance claims lasting more than
one year, representing 49% of claims; however, these
conditions are third most common (16%) for insurance
claims lasting one year or less, after cancer and
musculoskeletal conditions (31% and 25% of claims,
respectively).
922
Medicare Advantage plans are allowed to
provide supplemental benefits, such as adult day care,
caregiver support and in-home support services for
chronically ill beneficiaries. However, only 17% of
individual plans offered in-home support services as a
benefit in 2023, and these supplemental benefits are
unlikely to offset a substantial portion of long-term
care costs.
890
To address the dearth of private long-term care
insurance options and the high out-of-pocket cost of
long-term care services, Washington became the first
state in the country to create a public state-operated
long-term care insurance program.
925
The Long-Term
Services and Supports Trust Program (WA Cares Fund)
is funded by a payroll tax on employees of 58 cents per
$100 earned that began in July 2023, and self-employed
individuals can choose to participate in the program. The
program is currently structured to pay up to $36,500 in
lifetime benefits beginning in July 2026.
926
Although
other states have contemplated implementing a long-
term care tax to fund long-term care insurance, none
have yet passed legislation.
927
Medicaid Costs
Medicaid covers nursing home care and long-term
care services in the community for individuals who meet
program requirements for level of care, income and
assets.
928
To receive coverage, beneficiaries must have
low incomes. Beneficiaries with financial resources
above Medicaid thresholds may spend down their assets
and income to become eligible for coverage. Once
enrolled, most nursing home residents with Medicaid
must spend all of their Social Security income and any
other monthly income, except for a very small personal
needs allowance, to pay for nursing home care.
Medicaid only makes up the difference if the nursing
home resident cannot pay the full cost of care or has a
financially dependent spouse. Although Medicaid covers
the cost of nursing home care, its coverage of many other
long-term care and support services, such as assisted
living care, home-based skilled nursing care and help with
personal care, varies by state.
Twenty-four percent of older individuals with Alzheimer’s
or other dementias who have Medicare also have
Medicaid coverage, compared with 10% of individuals
without dementia.
863
Because Medicaid pays for nursing
home and other long-term care services, the high use
of these services by people with dementia translates
into high costs to Medicaid. Average annual Medicaid
payments per person for Medicare beneficiaries with
Alzheimer’s or other dementias ($6,771) were 22 times as
great as average Medicaid payments for Medicare
beneficiaries without Alzheimer’s or other dementias
($305) (see Table 15, page 72).
863
Much of the difference
in payments for beneficiaries with Alzheimer’s or other
dementias compared with other beneficiaries is due to
the costs associated with nursing home care.
Total Medicaid spending for people with Alzheimer’s or
other dementias is projected to be $68 billion in 2024.
A11
Actual and estimated state-by-state Medicaid spending
for people with Alzheimer’s or other dementias in
2020 and 2025 (in 2020 dollars) is included in Table 20,
page 83.
Use and Costs of Care at the End of Life
Hospice care provides medical care, pain management,
and emotional and spiritual support for people who
are dying, including people with Alzheimer’s or other
dementias, either in a care residence or at home.
Hospice care also provides emotional and spiritual
support and bereavement services for families of people
who are dying. The main purpose of hospice is to allow
individuals to die with dignity and without pain and other
distressing symptoms that often accompany terminal
illness. Medicare is the primary source of payment for
hospice care, but private insurance, Medicaid and other
sources also pay for hospice care. Medicare beneficiaries
enrolled in Medicare Part A (i.e., Medicare’s hospital
insurance) can choose to enroll in Medicare’s hospice
benefit if a hospice physician certifies that the individual
is terminally ill (i.e., expected to live six months or less),
and the individual accepts palliative or comfort care and
forgoes curative care for the terminal illness. In this way,
hospice care replaces other Medicare-covered benefits
for treating the terminal illness and related conditions.
929
Based on data from the National Hospice Survey for
2008 to 2011, nearly all hospices (99%) cared for

*All cost figures are reported in 2020 dollars.
Created from data from the Lewin Model.
A11
State
2020
(in millions
of dollars)
2025
(in millions
of dollars)
Percentage
Increase
Alabama $925 $1,127 21.8
Alaska 76 110 44.6
Arizona 414 545 31.7
Arkansas 396 454 14.6
California 4,197 5,235 24.7
Colorado 635 789 24.1
Connecticut 1,022 1,187 16.1
Delaware 253 313 23.6
District of Columbia 126 135 6.8
Florida 2,689 3,453 28.4
Georgia 1,265 1,594 26.0
Hawaii 240 285 18.7
Idaho 149 196 31.2
Illinois 1,787 2,199 23.1
Indiana 1,054 1,233 17.1
Iowa 676 792 17.2
Kansas 473 543 14.6
Kentucky 803 949 18.2
Louisiana 765 934 22.1
Maine 212 274 29.5
Maryland 1,231 1,535 24.7
Massachusetts 1,753 2,031 15.9
Michigan 1,487 1,738 16.9
Minnesota 905 1,087 20.1
Mississippi 606 729 20.4
Missouri 973 1,137 16.8
State
2020
(in millions
of dollars)
2025
(in millions
of dollars)
Percentage
Increase
Montana $166 $203 22.2
Nebraska 372 411 10.3
Nevada 203 277 36.5
New Hampshire 254 335 31.9
New Jersey 2,186 2,614 19.6
New Mexico 227 279 22.9
New York 5,453 6,306 15.6
North Carolina 1,332 1,628 22.2
North Dakota 190 215 13.2
Ohio 2,534 2,940 16.0
Oklahoma 516 611 18.3
Oregon 253 317 25.4
Pennsylvania 3,658 4,029 10.2
Rhode Island 470 565 20.1
South Carolina 652 818 25.4
South Dakota 182 212 16.6
Tennessee 1,109 1,377 24.2
Texas 3,202 3,949 23.3
Utah 185 235 27.0
Vermont 116 146 26.4
Virginia 1,000 1,266 26.6
Washington 547 689 26.0
West Virginia 445 521 17.1
Wisconsin 777 924 18.9
Wyoming 86 111 28.8
Total Medicaid Payments for Americans Age 65 and Older Living with Alzheimer’s or Other Dementias by State*
83Use and Costs of Health Care, Long-Term Care and Hospice
Table
20
84 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
individuals with dementia, and 67% of hospices had
residents with a primary diagnosis of dementia.
930
In
2017, 4,254 U.S. companies provided hospice care in
the home, assisted living communities, long-term care
residences, inpatient hospitals, and inpatient hospice
and other settings.
931
Nearly two-thirds (63%) of Medicare decedents
(i.e., people who have died) with Alzheimer’s or other
dementias used hospice in their last six months of life
in 2017 compared with 36% of Medicare decedents
without Alzheimer’s or other dementias.
932
In 2017,
dementia, including Alzheimer’s dementia, was the
second most common primary diagnosis for Medicare
beneficiaries using hospice care, representing 18% of
Medicare beneficiaries receiving hospice care (Table 21,
page 85).
931
Alzheimer’s or other dementias are even
more common in individuals receiving hospice care
when taking into account the disease as a coexisting
or secondary condition. Forty-five percent of hospice
users in 2020 had a diagnosis of Alzheimer’s or
other dementias.
893
Patterns of hospice use for individuals with dementia
differ from patterns for individuals without dementia in
at least two notable ways. The average number of days
of hospice care for individuals with a primary diagnosis
of dementia was 50% higher than for individuals with
other primary diagnoses, based on data from the 2008
to 2011 National Hospice Survey.
930
Individuals with a
primary diagnosis of dementia use an average of 112
days of hospice care versus 74 days for individuals with
other primary diagnoses. Recently, researchers found
that individuals with dementia as either the primary
hospice diagnosis or as a secondary condition were more
likely than other hospice users to be enrolled in hospice
for more than six months.
933
However, long hospice stays
place individuals with dementia at risk for disenrollment,
and researchers have found that individuals with
dementia are more likely to be disenrolled after more
than six months in hospice than patients with other
diagnoses.
930, 933
Reasons for disenrollment include
admission to an acute care hospital, loss of eligibility
because the individual was no longer terminally ill,
and failure to recertify for hospice.
934
For hospice
enrollments of at least six months, hospice providers are
required to assess individuals every 60 days, beginning
at six months, to ensure they continue to meet eligibility
requirements, and these assessments coupled with
Medicare payment rates that are roughly 20% lower
after the first 60 days, may contribute to disenrollment;
however, more research is needed to understand the
implications of these policies for individuals with
dementia in hospice.
935, 936
Overall, 12.2% of Medicare beneficiaries with
Alzheimer’s had at least one hospice claim in 2018,
compared with 1.4% of Medicare beneficiaries without
the disease, translating into per-person hospice
payments (for all beneficiaries, regardless of whether
they used any hospice services) of $2,321 for individuals
with Alzheimer’s compared with $136 for all other
Medicare beneficiaries.
863
In 2016, Medicare
reimbursement for home hospice services changed from
a simple daily rate for each setting to a two-tiered
approach that provides higher reimbursement for days
1 to 60 than for subsequent days and a service intensity
add-on payment for visits by a registered nurse or social
worker in the last seven days of life. In fiscal year 2024,
the routine home care rates are $218.33 per day for days
1 to 60 and $172.35 per day for days 61 and beyond.
935
Intensity of care at the end of life has decreased over
the past two decades as hospice enrollment has
increased. One group of researchers found that the
number of inpatient hospital days in the last six months
of life decreased from 15.3 to 11.8 between 2004 and
2017, although intensive care unit stays and number of
days in a skilled nursing facility increased modestly over
the same time period.
932
Expansion of hospice care is
associated with fewer individuals with dementia having
more than two hospitalizations for any reason or more
than one hospitalization for pneumonia, urinary tract
infection, dehydration or sepsis in the last 90 days of
life.
937
For Medicare beneficiaries with advanced
dementia who receive skilled nursing home care in the
last 90 days of life, those who are enrolled in hospice are
less likely to die in the hospital.
938
Additionally, those
enrolled in hospice care are less likely to be hospitalized
in the last 30 days of life
939
and more likely to receive
regular treatment for pain.
940
Satisfaction with medical
care is higher for families of individuals with dementia
who are enrolled in hospice care than for families of
individuals with dementia not enrolled in hospice care.
941
Despite the important role of end-of-life care for
individuals with Alzheimer’s, differences in hospice use
by race/ethnicity exist. One group of researchers found
substantially smaller proportions of Black and Hispanic
Medicare beneficiaries with dementia enrolled in hospice
in the last six months of life compared with White
Medicare beneficiaries with dementia (38% and 43%
versus 51% respectively).
942
Furthermore, larger
proportions of Black and Hispanic beneficiaries with
dementia had at least one emergency department visit
(80% and 77% respectively) and at least one
hospitalization (77% for both groups) compared with
White beneficiaries with dementia (71% and 68%
respectively) in the last six months of life.
942
Black and
Hispanic beneficiaries were also more likely to have an
emergency department visit and/or a hospitalization
after hospice enrollment.

Created from data from the U.S. Centers for Medicare & Medicaid Services.
931
State
Number of
Beneficiaries
Percentage of
Beneficiaries
Alabama 5,867 18
Alaska 95 14
Arizona 7,229 18
Arkansas 3,133 18
California 30,045 20
Colorado 3,254 15
Connecticut 2,380 15
Delaware 716 12
District of Columbia 263 18
Florida 19,897 15
Georgia 10,435 21
Hawaii 943 16
Idaho 1,566 17
Illinois 9,795 18
Indiana 5,922 17
Iowa 3,278 17
Kansas 2,770 18
Kentucky 2,895 15
Louisiana 4,786 19
Maine 1,494 19
Maryland 4,072 17
Massachusetts 7,245 23
Michigan 9,001 16
Minnesota 5,399 21
Mississippi 3,547 20
Missouri 5,991 17
State
Number of
Beneficiaries
Percentage of
Beneficiaries
Montana 507 11
Nebraska 1,648 18
Nevada 2,167 17
New Hampshire 1,007 17
New Jersey 8,207 23
New Mexico 1,523 15
New York 7,669 16
North Carolina 8,486 17
North Dakota 468 18
Ohio 12,656 17
Oklahoma 4,102 18
Oregon 3,565 17
Pennsylvania 12,384 17
Rhode Island 1,657 25
South Carolina 6,038 20
South Dakota 421 13
Tennessee 6,435 19
Texas 26,672 22
Utah 2,506 19
Vermont 543 17
Virginia 6,440 19
Washington 5,459 20
West Virginia 1,552 15
Wisconsin 5,086 16
Wyoming 89 7
U.S. Total 278,192 18
Number and Percentage of Medicare Beneficiaries Admitted to Hospice with a Primary Diagnosis of Dementia by State, 2017
85Use and Costs of Health Care, Long-Term Care and Hospice
Table
21

86 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Researchers have found similar reductions in
hospitalizations at the end of life for individuals
receiving palliative care. For nursing home residents
with moderate-to-severe dementia, those who received
an initial palliative care consultation between one and
six months before death had significantly fewer
hospitalizations and emergency department visits in the
last seven and 30 days of life compared with those who
did not receive palliative care.
943
Individuals with an initial
palliative care consultation within one month of death
also had significantly fewer hospitalizations in the last
seven days of life compared with those who did not
receive palliative care.
943
One essential component of
palliative care is advance care planning (i.e., a plan for
future medical care that includes the patient’s goals and
preferences, should the patient become unable to make
their own decisions). Although Medicare reimburses
physicians for visits related to advance care planning,
these visits rarely occur. In 2017, less than 3% of fee-for-
service Medicare beneficiaries had at least one claim for
advance care planning.
944
However, compared with
individuals without newly diagnosed conditions, Medicare
beneficiaries with newly diagnosed Alzheimer’s were 1.3
times as likely to have one or more claims for advance
care planning. Racial/ethnic disparities in the completion
of advance care planning in the last six months of life are
concerning. One group of researchers found that the
proportion of Black and Hispanic Medicare beneficiaries
with dementia with advance care planning was less than
half that of White beneficiaries.
942
Life-Sustaining Interventions at the End of Life
Life-sustaining interventions, such as mechanical
ventilation, tracheostomy, tube feeding and
resuscitation can be especially harmful to individuals
with Alzheimer’s. Although these interventions may not
be consistent with patient preferences, individuals with
Alzheimer’s may be at greater risk for receiving these
treatments. One group of researchers found that
Medicare beneficiaries with advanced dementia who
lived in the community were 1.8 times as likely to
receive life-sustaining treatments in the last three
months of life, compared with individuals without
dementia living in the community.
945
Individuals with
frequent transitions between health care settings are
more likely to have feeding tubes at the end of life, even
though feeding tube placement does not prolong life or
improve outcomes.
946
The odds of having a feeding tube
Created from data from the National Center for Health Statistics.
948,949
Nursing home/long-term care
Percentage
Decedent’s home Hospice facility
Medical facility Other Place of death not recorded
Place of Death Due to Alzheimer’s Disease, 2002 to 2021
100
90
80
70
60
50
40
30
20
10
0
02 03 04 05 06 07 08 09 10 11 12 13 14 15 16 17 18 19 20 21Year
Figure
15
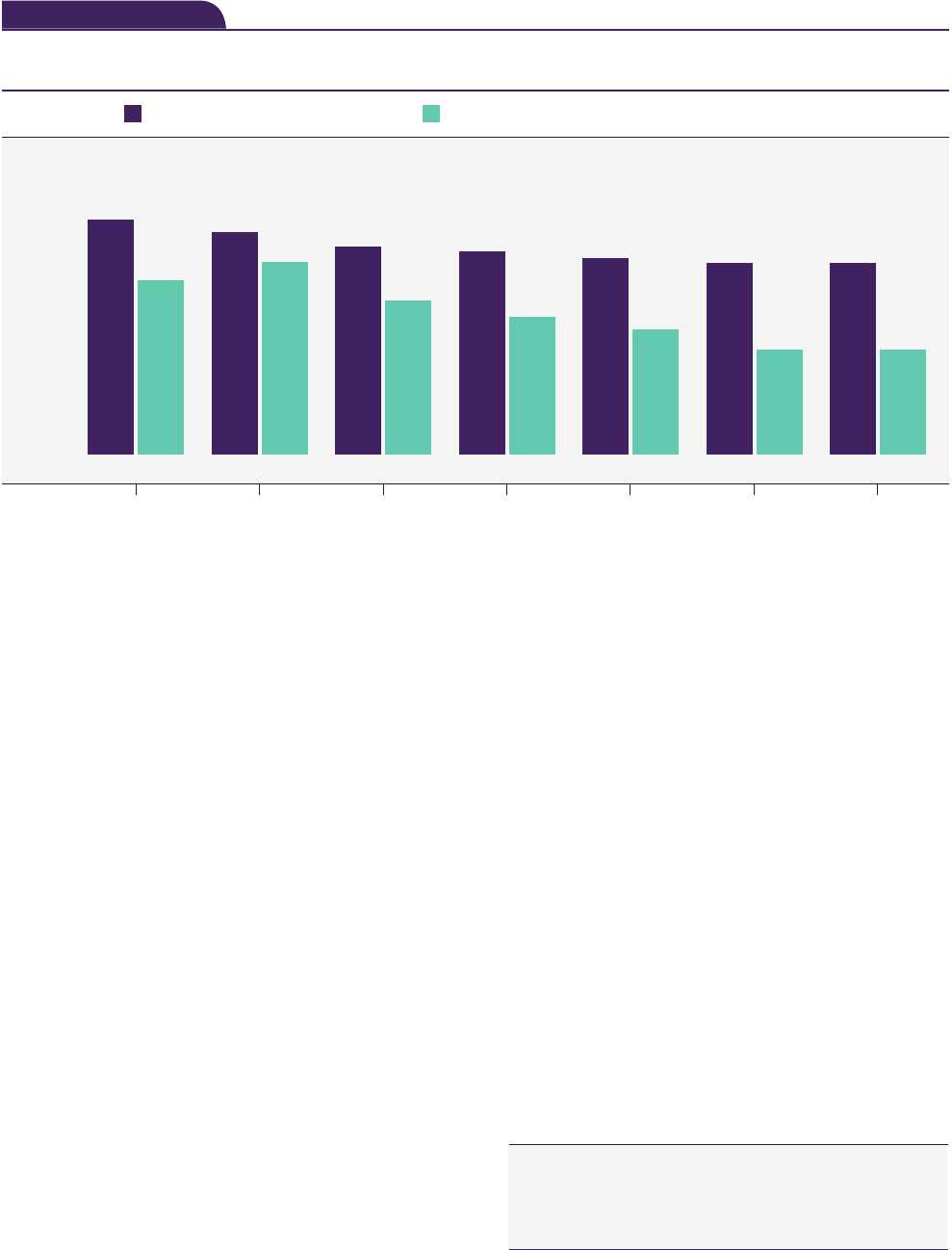
inserted at the end of life vary across the country and
are not explained by severity of illness, restrictions on
the use of artificial hydration and nutrition, ethnicity or
gender. With the expansion of Medicare-supported
hospice care, the use of feeding tubes in the last three
to six months of life has decreased for individuals with
Alzheimer’s or other dementias.
932, 937
Finally, with the
increased focus on the lack of evidence supporting
feeding tube use for people with advanced dementia,
the proportion of nursing home residents receiving a
feeding tube in the 12 months before death decreased
from nearly 12% in 2000 to less than 6% in 2014.
947
However, individuals with advanced dementia are
significantly more likely to receive tube feeding in
the last three months of life compared with those
without dementia.
945
Place of Death for Individuals with Alzheimer’s Disease
Between 2002 and 2021, the proportion of individuals
with Alzheimer’s who died in a nursing home decreased
from 67% to 42%, and the proportion who died in a
medical facility decreased from 13% to 5%. During the
same period, the proportion of individuals who died at
home increased from 15% to 37% (Figure 15).
948, 949
Use and Costs of Health Care and Long-Term
Care Services by Race and Ethnicity
Among Medicare beneficiaries with Alzheimer’s or other
dementias, Black beneficiaries had the highest unadjusted
Medicare payments per person per year, while White
beneficiaries had the lowest payments ($27,814 versus
$22,306, respectively) (Table 22, page 88). The largest
difference in payments was for hospital care, with Black
Medicare beneficiaries incurring 1.6 times as much in
hospital care costs as White beneficiaries ($9,006 versus
$5,791).
413
White beneficiaries had the highest hospice
payments, however, of all racial and ethnic groups. A study
of racial and ethnic differences in health care spending
using the Medical Expenditure Panel Survey found similar
patterns in unadjusted total spending.
950
However, after
adjusting for socioeconomic characteristics and functional
status, total health care spending did not differ significantly
among groups.
Created from unpublished data from the National 100% Sample Medicare Fee-for-Service Beneficiaries for 2019.
413
Congestive
heart failure
CancerCondition Chronic
kidney
disease
Coronary
artery
disease
Stroke DiabetesChronic
obstructive
pulmonary
disease
With Alzheimer’s or other dementias
Hospital stays Without Alzheimer’s or other dementias
Number of Hospital Stays per 1,000 Medicare Beneficiaries Age 65 and Older with Specified
Coexisting Medical Conditions, with and without Alzheimer’s or Other Dementias, 2019
1,000
800
600
400
200
0
816
606
774
668
722
536
706
478
682
436
666
368
666
366
87Use and Costs of Health Care, Long-Term Care and Hospice
This report keeps the racial, ethnic and other
population identifiers used in source documents
when describing findings from specific studies.
Figure
16

88 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
The COVID-19 pandemic has disproportionately
affected Americans living with Alzheimer’s and
other dementias.
As data continue to emerge on the toll of the pandemic, it is
increasingly clear that these individuals are more susceptible
both to contracting COVID-19 and developing severe illness
due to COVID-19. Individuals living and working in care
communities have been extremely vulnerable to COVID-19
due to the communal nature of these settings. Overall,
21% of all U.S. COVID-19 deaths occurred in either residents
or staff of long-term care facilities.
961
Through November 2021, of all people with fee-for-
service Medicare coverage who were hospitalized due to
COVID-19, 27% had a diagnosis of Alzheimer’ disease or
another dementia.
962
Even after adjusting for demographic
characteristics and other COVID-19 risk factors (including
living in long-term care or other care communities),
individuals with Alzheimer’s were at higher risk for
contracting and dying of COVID-19.
963, 964
One study using
data from electronic health records and adjusting for
COVID-19 risk factors found that individuals with
Alzheimer’s had twice the odds of being diagnosed with
COVID-19 as individuals without Alzheimer’s. The risk was
even higher for Black adults with dementia, who had nearly
three times the odds of contracting COVID-19 compared
with White adults with dementia.
964
Another study using
In a study of Medicare-Medicaid dually eligible
beneficiaries diagnosed with Alzheimer’s dementia,
researchers found significant differences in the costs of
care by race and ethnicity.
951
These results demonstrated
that Blacks had significantly higher costs of care than
Whites or Hispanics, primarily due to more inpatient care
and more comorbidities. These differences may be
attributable to later-stage diagnosis, which may lead to
higher levels of disability while receiving care; delays in
accessing timely primary care; lack of care coordination;
duplication of services across providers; or inequities in
access to care. However, more research is needed to
understand the reasons for this health care disparity.
Use of Potentially Avoidable Health
Care Services
Preventable Hospitalizations and Emergency
Department Care
Preventable hospitalizations are one common measure
of health care quality. Preventable hospitalizations are
hospitalizations for conditions that could have been
avoided with better access to, or quality of, preventive and
primary care. Unplanned hospital readmissions within 30
days are another type of hospitalization that potentially
could have been avoided with appropriate post-discharge
care. In 2013, 21% of hospitalizations for fee-for-service
Medicare enrollees with Alzheimer’s or other dementias
Medicare claims data similarly found that beneficiaries
with a diagnosis of dementia were 50% more likely to be
diagnosed with COVID-19 and 60% more likely to die of
COVID-19 than were beneficiaries without dementia, after
adjusting for COVID-19 risk factors.
963
Evidence is still emerging on how health care utilization
changed during the pandemic for individuals with
Alzheimer’s and other dementias. For example, one area of
concern is the effect of not receiving some types of health
care because of service and other limitations related to
COVID-19. However, we do know that individuals
diagnosed with dementia had the highest rates of
hospitalization for COVID-19 compared with individuals
with any of the 20 other common chronic conditions
analyzed (including chronic kidney disease, diabetes,
hypertension and obesity) in 2020.
965
This risk was not
limited to congregate settings such as assisted living
residences and nursing homes. Individuals with a diagnosis
of Alzheimer’s who were living in the community were
more than 3.5 times as likely to be hospitalized for
COVID-19 as individuals without Alzheimer’s who were
living in the community.
965
The COVID-19 Pandemic and Health Care Utilization and Costs

Created from unpublished data from the National 100% Sample Medicare Fee-for-Service Beneficiaries for 2019.
413
Race/Ethnicity
Total Medicare
Payments
Per Person Hospital Care Physician Care
Skilled
Nursing Care
Home
Health Care Hospice Care
White $22,306 $5,791 $3,725 $3,297 $1,912 $4,137
Black 27,814 9,006 4,528 4,338 1,970 2,910
Hispanic 25,729 7,836 4,298 3,763 2,371 3,416
Other 22,864 7,260 3,917 3,663 1,959 2,817
Average Annual Per-Person Payments by Type of Service and Race/Ethnicity for Medicare
Beneficiaries Age 65 and Older, with Alzheimer’s or Other Dementias, in 2023 Dollars
89Use and Costs of Health Care, Long-Term Care and Hospice
were either for unplanned readmissions within 30 days or
for an ambulatory care-sensitive condition (a condition
that was potentially avoidable with timely and effective
ambulatory — that is, outpatient — care).
952
The total
cost to Medicare of these potentially preventable
hospitalizations was $4.7 billion (in 2013 dollars; $6.1 billion
in 2023 dollars).
952
Of people with dementia who had at
least one hospitalization, 18% were readmitted within
30 days; and of those who were readmitted within 30 days,
27% were readmitted two or more times.
952
Ten percent
of Medicare enrollees had at least one hospitalization
for an ambulatory care-sensitive condition, and 14% of
total hospitalizations for Medicare enrollees with
Alzheimer’s or other dementias were for ambulatory
care-sensitive conditions.
952
Based on Medicare administrative data from 2013 to 2015,
23.5% of diagnosed individuals with Alzheimer’s or other
dementias had at least one preventable hospitalization.
953
Black older adults had a substantially higher proportion of
preventable hospitalizations (31%) than Hispanic and White
older adults (22% for each group).
Based on data from the Health and Retirement Study (HRS)
and Medicare, after controlling for demographic variables,
clinical characteristics (e.g., presence of chronic medical
conditions, number of hospitalizations in the prior year) and
health risk factors, individuals with dementia had a 30%
greater risk of having a preventable hospitalization than
those without a neuropsychiatric disorder (that is,
dementia, depression or cognitive impairment without
dementia).
954
Moreover, individuals with both dementia and
depression had a 70% greater risk of preventable
hospitalization than those without a neuropsychiatric
disorder.
954
Another group of researchers found that
individuals with dementia and a caregiver with depression
had 73% higher rates of emergency department use over
six months than individuals with dementia and a caregiver
who did not have depression.
955
Medicare beneficiaries who have Alzheimer’s or other
dementias and a serious coexisting medical condition (for
example, congestive heart failure) are more likely to be
hospitalized than people with the same coexisting medical
condition but without dementia (Figure 16, page 87).
413
One
research team found that individuals hospitalized with heart
failure are more likely to be readmitted or die after hospital
discharge if they also have cognitive impairment.
956
Another
research team found that Medicare beneficiaries with
Alzheimer’s or other dementias have more potentially
avoidable hospitalizations for diabetes complications and
hypertension, meaning that the hospitalizations could possibly
have been prevented through proactive care management in
the outpatient setting.
957
A third research team found that
having depression, rheumatoid arthritis or osteoarthritis was
associated with higher emergency department use in
Medicare beneficiaries with possible or probable dementia
and two or more other chronic conditions.
958
Differences in health care use between individuals with and
without dementia are most prominent for those residing in
the community. Based on data from the HRS, community-
residing individuals with dementia were more likely to have a
potentially preventable hospitalization, an emergency
department visit that was potentially avoidable, and/or an
emergency department visit that resulted in a hospitalization
than community-residing individuals without dementia.
959
For individuals residing in a nursing home, there were no
differences between those with and without dementia in the
likelihood of being hospitalized or having an emergency
department visit.
Table
22
90 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Health Care Delivery Models with Skilled
Nursing Facilities
Changes in health care delivery and payment models, such
as the integration of care across different health care
settings and the structure of health care payments, may
impact health care utilization for individuals with
Alzheimer’s disease or other dementias. Research has
shown modest differences in outcomes for skilled nursing
facilities that share providers with at least one hospital
versus those that have dedicated providers within the
skilled nursing facilities. An analysis of Medicare claims data
for 2008 to 2016 showed that skilled nursing facilities that
shared providers with at least one hospital were more likely
to have an Alzheimer’s unit, had fewer 30-day
readmissions, and had more patients successfully
discharged to the community. The skilled nursing facilities
that maintain these relationships have modestly better
outcomes,
960
although there has been a decline in hospital-
skilled nursing facility linkages in the past two decades due
to a shift toward dedicated hospitalists and skilled nursing
facility providers.
Looking to the Future
Absent additional treatment breakthroughs, total
annual payments for health care and long-term care for
people with Alzheimer’s or other dementias are projected
to increase from $360 billion in 2024 to just under
$1 trillion in 2050 (in 2024 dollars). This dramatic rise
includes nearly three-fold increases both in government
spending under Medicare and Medicaid and in out-of-
pocket spending.
A11
Concurrent with this large projected
increase, the Medicare Hospital Insurance Trust Fund,
which covers spending for Medicare Part A (hospital care),
is projected to go into a deficit, based on projections of
growth, overall health care spending trends and
population aging.
966
Potential Impact of Changing the Trajectory of
Alzheimer’s Disease
While there are currently no treatments approved by the
U.S. Food and Drug Administration (FDA) that prevent or
cure Alzheimer’s disease, two drugs that change the
underlying biology of Alzheimer’s disease (aducanumab and
lecanemab) have recently been approved. They were tested
in people with confirmed beta-amyloid accumulation in the
brain who were living with MCI due to Alzheimer’s disease
or mild dementia due to Alzheimer’s. Several other
treatments that target beta-amyloid accumulation and
other well-established brain changes of Alzheimer’s disease
are in late-stage development. These treatments are
promising for changing the course of the disease.
Although these treatments, and others on the horizon,
have the potential to improve quality of life for millions of
adults and their families, there are some considerations.
For example, while lecanemab demonstrated clinically
significant changes in cognition and function, its effects
may be imperceptible to those being treated.
967
Additionally, there is an increased risk of adverse events
with lecanemab and other anti-amyloid therapies, including
amyloid-related imaging abnormalities with edema or
effusions. Another concern is the affordability of treatment
to both payers, such as Medicare, and to individuals and
their families, who may bear out-of-pocket costs due to
deductibles, copayments and coinsurance.
968
Additionally,
the current market price of treatment is high, at $26,500
per person per year.
969, 970
Lack of affordability of Medicare
supplemental insurance is also likely to widen disparities in
access to treatment for Medicare enrollees with low
incomes given these market prices.
From a societal perspective, the number of people
potentially eligible and the total cost of these treatments is
a potential concern. The Centers for Medicare & Medicaid
Services covers Medicare beneficiaries diagnosed with MCI
or Alzheimer’s dementia, and has a physician participating
in a registry for these treatments.
971
Although aducanumab
and lecanemab are for individuals with mild Alzheimer’s
dementia and MCI due to Alzheimer's disease, the actual
number of people who may be eligible is projected to be
much smaller. One group of researchers applied the clinical
trial eligibility criteria to a sample of adults with dementia
or MCI and a positive brain amyloid PET scan and found
that only 8% of the sample would meet the lecanemab
clinical trial inclusion and exclusion criteria.
972
Before the approval of aducanumab and lecanemab,
several groups of researchers had estimated the health
and long-term care cost implications of hypothetical
interventions that either slow the onset of dementia or
reduce the symptoms.
431, 973-975
One analysis assumed a
treatment that delayed onset of Alzheimer’s by five years
would reduce total health and long-term care spending for
people with Alzheimer’s by 33%, including a 44% reduction
in out-of-pocket payments by 2050,
973
and another study
projected a 14% reduction in total health care spending for
people age 70 and older with Alzheimer’s from a one-year
delay, a 27% reduction from a three-year delay and a 39%
reduction from a five-year delay by 2050.
974
Beyond the
single-year costs, the study also found that a delay in onset
may increase total lifetime per capita health care spending
due to longer life associated with delaying the onset of
dementia, although the additional health care costs may be
offset by lower informal care costs. Finally, a third study
estimated that a treatment slowing the rate of functional
decline among people with dementia by 10% would reduce
91Use and Costs of Health Care, Long-Term Care and Hospice
total average per-person lifetime costs by $3,880 in 2015
dollars ($4,759 in 2023 dollars), while a treatment that
reduces the number of behavioral and psychological
symptoms by 10% would reduce total average per-person
lifetime costs by $680 ($834 in 2023 dollars).
431
However,
these studies did not take into account the current market
price for FDA-approved drugs.
Therapies that change the course of the disease may not
be the only way to reduce health and long-term care costs.
The Alzheimer’s Association commissioned a study of the
potential cost savings of early diagnosis,
975
assuming that
88% of individuals who will develop Alzheimer’s disease
would be diagnosed in the MCI phase rather than the
dementia phase or not at all. Approximately $7 trillion could
be saved in medical and long-term care costs for individuals
who were alive in 2018 and will develop Alzheimer’s
disease. Cost savings were the result of (1) a smaller spike in
costs immediately before and after diagnosis during the
MCI phase compared with the higher-cost dementia phase,
and (2) lower medical and long-term care costs for
individuals who have diagnosed and managed MCI and
dementia compared with individuals with unmanaged MCI
and dementia.
The savings from a treatment or an earlier diagnosis may
depend on structural changes to the health care system.
Capacity constraints — such as a limited number of
qualified providers and facilities — could severely restrict
access to new treatments.
976, 977
For example, modeling by
the RAND Corporation in 2017 showed that with an
anti-amyloid therapy for people in the MCI and early
dementia stages of the disease, approximately 2.1 million
individuals with MCI due to Alzheimer’s disease would
develop Alzheimer’s dementia between 2020 and 2040
while on waiting lists for treatment. This model assumed
that the hypothetical treatment would require infusions at
infusion centers and PET scans to confirm the presence of
amyloid in the brain to support initiation of treatment with
an anti-amyloid medication.

Special Report
Mapping a Better Future for
Dementia Care Navigation

93
“Following a dementia diagnosis too many individuals
and families are left on their own groping in the dark
for services that can help them. I don’t want others to
go through what I did. I lost two to three years searching
for answers. It was time I could have spent differently.”
— Pamela, individual living with early-onset Alzheimer’s disease
Today's Dementia Care
978
Dementia care is a complex maze encompassing
interactions with primary care providers,
specialists (including those involved in managing
chronic conditions coexisting with cognitive
issues), social services, medication management
and caregiver support (Figure 17).
978
Navigating this maze is difficult and often frustrating for
people living with Alzheimer's or other dementia and their
caregivers. Without a clear path forward, any roadblock or
detour along the way can have considerable ramifications.
This could materialize as delayed detection, diagnosis
and treatment of early-stage cognitive issues or mild
cognitive impairment (MCI). For caregivers, a detour could
cause them to miss valuable or necessary educational
opportunities, miss connections with community-based
services for respite and behavioral health support or be
unable to locate resources that could help reduce their
stress. Breakdowns in transitions of care between
health care providers and settings limit high-quality,
comprehensive and/or appropriate dementia care, as
evidenced by an increase in emergency room visits and
hospitalizations and decreased quality of life.
979
Today’s
Dementia Care
Patient
+
Caregiver
Specialist
Social Services
Primary Care Provider
Medication
Caregiver Supports
Figure
17
Special Report - Mapping a Better Future for Dementia Care Navigation

94 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Caregiver Burden and Stress Are Compounded
by the Complexity of Dementia Care
Unpaid caregivers (sometimes referred to as care partners),
who can be a spouse, family member or friend, provide
extensive, sometimes all-encompassing care for people
living with Alzheimer's or other dementia. In 2023,
11.5 million family members and other caregivers of
people living with Alzheimer's or other dementia provided
an estimated 18.4 billion hours of unpaid help. On average,
this represents nearly 31 hours of care per caregiver per
week or 1,612 hours per caregiver per year.
A8
Caregivers
spend much of this time interacting with the health care
system or learning more about dementia caregiving, and
nearly 2 in 3 (63%) help with health or medical care.
427, 440
Daily health care activities may include scheduling
appointments with health care providers, attending
doctor's visits, and scheduling social and community
support for themselves and the person living with
dementia, such as in-home assistance, adult day programs
or meal delivery. Collectively, performing these activities
and the organization of care across multiple health care
providers can be described as care coordination.
980, 981
The effort expended trying to find their way through the
health care system can add to the already high emotional
and physical stress levels that caregivers experience.
Caregivers need assistance to gather dementia care
information, synthesize it and act upon it in a way that
does not add to their stress level — support that primary
care providers and health systems have historically
been ill-equipped or unprepared to provide. (For more
information on supporting people living with Alzheimer’s
or other dementia and the impact of unpaid caregiving,
see the Caregiving section, page 42.)
Nationwide Movement to Improve Care
While Reducing Strain on Caregivers
For more than a decade, the National Plan to Address
Alzheimer's Disease has included goals to improve health
care quality and expand support for individuals living with
Alzheimer’s disease or other dementia and their families.
982
Recently, the Alzheimer’s Association and the Alzheimer's
Impact Movement (AIM) — a separately incorporated
advocacy affiliate of the Alzheimer's Association —
championed critical legislation to help unravel the health
care maze.
983
This legislation, the bipartisan Comprehensive
Care for Alzheimer's Act, proposed a new approach to
dementia care management covering care coordination
and navigation, caregiver education and support, and
alternative payment models for physician
reimbursement.
983, 984
In July 2023, the Centers for Medicare & Medicaid Services
(CMS) announced the culmination of policy, working group
and legislative efforts like the Comprehensive Care for
What is GUIDE?
986
The Guiding an Improved Dementia Experience
(GUIDE) Model is an eight-year pilot program in
dementia care management designed to help dementia
patients and caregivers better navigate health care and
social support systems to improve dementia care.
Three primary aims of GUIDE are to:
• Improve quality of life for people living with
dementia.
• Reduce strain on their unpaid caregivers.
• Enable people living with dementia to remain in
their homes and communities.
Beginning in July 2024, health care providers
who participate in GUIDE will deliver supportive
services to people living with dementia, including
comprehensive, person-centered assessments and
care plans, care coordination and 24/7 access to a
support line. They will also provide access to a care
navigator to help patients and caregivers access
services and support.
GUIDE acknowledges that current fee-for-service
payment structures prevent many practices from
implementing sustainable dementia care management
programs.
984-986
To overcome this challenge, GUIDE
is testing an alternative payment model (APM) to
incentivize health systems and increase the likelihood
that smaller practices, rural practices and inner-city
health centers that traditionally do not have the
financial resources of larger entities will be able to
deliver this type of program.
984, 986
The APM shifts
payments from a fee for individual services to a
monthly per-patient payment for all services under the
GUIDE Model umbrella, including those not typically
reimbursed by Medicare.
984, 986
Recognizing that some health care providers will face
resource, staffing and capability constraints, a second
GUIDE Model track will engage those who do not have
experience offering comprehensive dementia care
services. The Centers for Medicare & Medicaid Services
will offer these organizations technical assistance,
learning support and a preparatory pre-implementation
year to facilitate their participation in the model. (For
more information about GUIDE and other national
strategies to support caregivers, see the Caregiving
section, page 42, and the Workforce section, page 58.)
Alzheimer’s Act with the introduction of the Guiding an
Improved Dementia Experience (GUIDE) Model.
985
The new
model represents a pivotal opportunity to reshape and
enhance dementia care in the United States.
95
Navigators as Dementia Health Care Wayfinders
What is Care Navigation?
Care navigation is a critical process that assists patients and
caregivers with various aspects of obtaining health care,
such as helping them understand and overcome the
complex logistics of the health care system.
987-989
The
concept originated in cancer clinics in the 1990s to address
the overwhelming experience cancer patients faced trying
to manage their care.
989
It has since expanded to support
individuals with chronic diseases, including kidney disease,
diabetes and dementia.
987, 990-992
This assistance aims to be holistic and spans both medical
and nonmedical needs. On the medical side, care navigation
encompasses:
988, 991
• Scheduling appointments.
• Coordinating diagnostic testing or follow-ups.
• Providing disease education.
• Facilitating communication and referrals across clinical
specialties and organizations.
• Offering insurance and benefits assistance.
• Medication management.
Beyond medical support, care navigation programs may
connect patients and caregivers to nonmedical resources,
such as education, social services and community support.
991
Providing both types of navigation services underscores
the importance of a comprehensive approach to health
care.
991
By addressing medical and nonmedical needs, care
navigation programs strive to ensure better access to
health care, improve health outcomes and reduce
disparities in care.
990-992
What is Dementia Care Navigation?
Dementia care navigation shares many features of care
navigation programs in other specialties. However,
dementia care navigation emphasizes person-centered,
empowered support throughout the dementia care
journey.
990
This includes addressing nonmedical needs
unique to dementia care, such as behavioral symptom
management and access to community-based services and
supports for individuals with dementia and their
caregivers.
987, 990, 992, 993
The approach remains nimble to
adapt to emerging treatments and diagnostic tests, disease
progression, or other individual needs as they arise.
A key tenet of dementia care navigation is recognizing
the importance of the “care dyad”— the partnership
between people living with Alzheimer’s disease or other
dementia and their caregivers — in all aspects of dementia
care.
987, 992
As described previously, caregivers are prone to
information overload and may feel lost and overwhelmed
as they try to find their way to quality dementia care for
the person living with dementia. Dementia care navigation
programs are primed to offer assistance in this area.
In 2023, an expert workgroup convened by the
Alzheimer’s Association defined dementia care navigation
as “a program that provides tailored, strengths-based
support to persons living with dementia and their care
partners across the illness continuum and settings to
mitigate the impact of dementia through collaborative
problem solving and coaching.”
990
The workgroup outlined seven essential principles for
dementia care navigation, which underscore person-
centered care. According to the workgroup, dementia care
navigation should:
990, 993
1. Be person- and family-centered to ensure collaboration
and enhance engagement.
2
. Be culturally responsive and address disparities in access
to health care and support services.
3.
Include well-defined roles and responsibilities for all
members of the dementia care navigation team.
4.
Address barriers relating to medical, legal, financial,
emotional and other domains facing the person living
with dementia and their care partners.
5.
Provide coaching, education, and coordination in a
manner that is empowering, solution-focused and
strengths-based.
6.
Focus on the family unit as defined by the person living
with dementia.
7.
Ensure processes and protocols are evidence-based.
By adhering to these principles, dementia care navigation
programs can achieve more coordinated care for patients.
Health systems are already finding that dementia care
navigation can improve health outcomes, decrease the
number of emergency room visits, lower hospital
readmissions, shorten hospital stays and minimize delays
in long-term care placement.
993
What is a Care Navigator?
Care navigators are staff who guide patients and caregivers
through the health care system and help overcome barriers
that prevent them from getting the care they need.
991, 995
As integral members of interprofessional care teams, care
navigators are connectors — liaising, communicating and
facilitating medical and nonmedical needs.
991, 992, 995
Unlike
other care team members, their work spans various settings,
making them crucial touchpoints for care coordination.
991, 992
Common synonyms for “care navigator” include patient
navigator, care consultant and care team coordinator.
990, 992, 995
“ Due to high incidence, duration
and medical-social complexity,
dementia is an ideal candidate for
patient-centric health care delivery
models such as care navigation.”
990
Special Report - Mapping a Better Future for Dementia Care Navigation

96 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Prioritizing Person-Centered Care
in Dementia Care Navigation
990, 992-994
Person-centered care is the foundation of quality
dementia care. It challenges the traditional medical
model of care that tends to focus on processes,
schedules, and staff and organizational needs. Instead,
person-centered care stresses knowing the person
living with dementia, including their values, beliefs,
interests, abilities, likes and dislikes — both past
and present. A person-centered approach to care
assures the individual living with dementia and their
caregivers that health professionals know the
person, understand the person’s unique needs and
circumstances, and put these needs at the forefront in
making decisions and directing the person’s care.
Anchoring dementia care navigation in the principles
of person-centered care prioritizes the humanity of
each individual living with dementia while also
committing to a standard of care that elevates their
dignity, autonomy and quality of life at every stage.
Qualifications, training and time dedicated to the care
navigator role vary based on the care team structure and
the health system. They range from paraprofessionals to
licensed health care professionals, including nurses,
physician assistants, social workers, community health
workers or even former caregivers.
991
Care navigators,
including dementia care navigators, frequently share the
racial, ethnic or cultural background of those they assist,
enhancing the delivery of culturally competent care and
building stronger patient-navigator relationships.
987, 991
Dementia care navigators work with care dyads, but
evidence suggests that the primary recipients of navigation
services are caregivers.
987
In addition to the typical
navigation services, dementia caregivers frequently look to
care navigators for emotional support.
987
Through these
interactions, navigators and caregivers establish trusting,
long-term relationships.
In summary, care navigators are pivotal in helping patients
and caregivers find their way through an increasingly
intricate dementia care landscape.
Awareness and Understanding of Dementia
Care Navigation: Caregiver and Health Care
Workforce Surveys
This year’s Special Report takes a deeper look into how
dementia caregivers interact with the health care system
and how the non-physician health care workforce currently
employs care navigation. To better understand these
aspects of dementia care, the Alzheimer’s Association
commissioned Versta Research to conduct surveys of
(1) current or recent caregivers of adults age 50 or older
with cognitive issues (referred to in this report as dementia
caregivers) and (2) health care workers who are likely to
assume care navigation responsibilities in their role,
including nurses, social workers, and community health
workers (referred to in this report as the non-physician
health care workforce).
Key Findings
Dementia Caregivers
Dementia caregivers experience difficulty and stress
interacting with the health care system.
• Seven in 10 dementia caregivers (70%) report that
coordinating care is stressful. More than half of
caregivers (53%) said navigating health care is difficult.
Two in 3 dementia caregivers (66%) also have difficulty
finding resources and support for their needs.
Cost and care coordination are top stressors for
dementia caregivers.
• Two in 5 caregivers (42%) cite cost as a stressor in
getting care for their recipient. More than 1 in 3
caregivers report coordinating care with multiple
doctors (36%), securing appointments (35%) and getting
help taking a break (35%) as leading stressors in
navigating care for their recipient.
• Despite these and other stressors, only half of the
caregivers (51%) report ever talking with a health care
professional to help address their challenges.
Care navigation is an unfamiliar term for most
dementia caregivers, although many receive help akin
to care navigation.
• Three in 4 dementia caregivers (75%) report little or
no familiarity with the term “care navigator.” Half of
caregivers (50%) say they receive help with dementia
health care, support and services for the care recipient
from someone within their physician’s office or hospital.
• Nurses (42%) and social workers (35%) most often
provide navigation help to dementia caregivers.
97
Overwhelmingly, caregivers would welcome dementia
care navigator support and believe it would benefit both
the person living with dementia and the caregiver.
• More than 4 in 5 dementia caregivers (85%) say having
access to a care navigator would influence their choice
of dementia health care provider for the person they
care for.
• Three in 5 dementia caregivers (61%) cite improvement
in quality of life for their care recipient as a benefit of
having a care navigator. Two in 5 caregivers (43%)
believe access to a care navigator would improve the
overall health of their care recipient.
• Three in 5 dementia caregivers say less stress (62%)
and more peace of mind (62%) would be valuable
outcomes of having a care navigator. More than half
(56%) say having a care navigator could help them be
better caregivers.
Top services that would be helpful to dementia caregivers
include around-the-clock support, care coordination and
help understanding their care recipient’s condition.
• The vast majority of dementia caregivers (97%) say
they would find navigation services helpful.
• Nearly 2 in 5 dementia caregivers (36%) say a 24/7
helpline would be valuable in helping navigate care for
someone living with Alzheimer’s or other dementia.
Coordinating care and communication between
different specialists (34%) and getting help in
understanding their care recipient's condition (34%)
are also viewed as valuable services.
• Almost 1 in 3 dementia caregivers say it would be
helpful to have assistance with insurance or public
benefits (32%), scheduling appointments (31%), caregiver
training (31%), managing behavioral symptoms (31%),
understanding the health care system (30%) and finding
services to help with respite care (30%).
• The most helpful community-based resources cited
to help dementia caregivers include local caregiver
support groups (41%), respite programs (38%) and
availability of financial resources in the community (37%).
Non-Physician Health Care Workforce
The findings below reflect the views of the non-physician
health care workforce currently providing navigator-type
services to patients and caregivers in addition to the
other responsibilities of their role. The health care
workers surveyed included medical professionals (nurse
practitioners, physician assistants and registered nurses)
and nonmedical professionals (health care social workers,
community health workers and home health aides).
Most health care workers who provide navigator-type
services are familiar with the concept of care navigation,
even if that is not their focus.
• Three in 4 survey respondents (77%) are familiar with
the term “care navigator.” They spend roughly half their
time providing navigator-type services, even if they do
not refer to themselves as care navigators.
• Nearly 2 in 3 survey respondents (62%, predominantly
nonmedical professionals) help people living with
Alzheimer’s or other dementia and caregivers
understand the health care system, and more than 1 in
2 health care workers (57%) say they coordinate care
and communication with specialists.
• The most frequently provided navigator services are
referrals to community support services and resources
(75%), helping with emotional and cultural support
(68%), and screening for safety needs (66%).
Most health care workers providing navigator-type
services have experience in other medical specialties,
with few focusing exclusively on dementia.
• Four in 5 survey respondents (80%) have navigation
experience in non-dementia medical specialties, and
fewer than 1 in 10 (7%) focus primarily on offering
navigator-type support and services to people living
with dementia.
• Most providing navigation services (93%) feel at least
somewhat knowledgeable about MCI, Alzheimer’s
disease and other dementia but only 1 in 3 (36%) report
they are very knowledgeable.
• Nearly 9 in 10 (86%) feel knowledgeable about directing
patients with dementia and caregivers to appropriate
health care resources, but less than 1 in 3 (30%) feel
very knowledgeable. Four in 5 (82%) feel knowledgeable
about directing patients with dementia and caregivers
to community resources, but only 31% say they are very
knowledgeable.
Training in dementia care navigation is lacking and not
standardized.
• Three in 4 health care workers providing care navigation
(75%) indicate they received no formal training in
dementia care navigation.
• Those who did receive training were predominantly
nonmedical professionals, receiving a median of
30 hours of formal training. Medical professionals
who were trained received a median of 20 hours of
formal training.
Nonmedical professionals are viewed as best suited to help
people with dementia and their caregivers navigate care.
• Nine in 10 health care workers offering navigation
support (92%) say social workers, community health
workers or home health aides are best suited to help
people living with dementia and their caregivers
navigate health care.
Special Report - Mapping a Better Future for Dementia Care Navigation
98 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Health care workers say more can be done to help
patients and families navigate dementia care but point out
current barriers.
• Six in 10 survey respondents (60%) believe that the
U.S. health care system is not effectively helping
patients and their families navigate dementia care.
• Nearly half surveyed (46%) say their organizations do
not have a clearly defined process for care coordination
and clinical pathways for patients with MCI, Alzheimer’s
disease or other dementia.
• More than 3 in 4 (77%) identified a lack of community-
based resources as a barrier, and 44% viewed it as the
greatest barrier. Seven in 10 (70%) called out current
reimbursement as a barrier, with 41% saying this was
the greatest barrier.
• Nearly 9 in 10 (87%) say developing alternative
payment models is important in providing future care
coordination for people diagnosed with dementia.
Survey Design and Research Methods
The surveys were designed to elicit in-depth responses
from both dementia caregivers and the non-physician
health care workforce about the current state and
challenges of navigation in dementia care.
The dementia caregiver survey analyzed distinct aspects
of the caregiving journey, including:
• Time spent on caregiving and top stressors.
• Challenges in navigating health care services.
• Challenges in locating or accessing community
supports and services.
• Awareness of dementia care navigators and/or
navigation programs.
• Which health care workers help with care navigation.
• Communication preferences for care navigation.
• Value of navigation services and community-based
resources.
• Anticipated benefits and outcomes of care navigation.
The non-physician health care workforce survey covered
various aspects of care navigation, including:
• Familiarity with navigator terminology.
• Focus areas for care navigation services being delivered.
• Frequency and preferred method of communication.
• Perceived value of care navigation.
• Which health care workers deliver navigation services.
• Barriers to care navigation.
• Background and training in care navigation.
Dementia Caregiver Survey
A survey of 1,533 U.S. adults who were current or recent
unpaid caregivers for a relative or friend age 50 or older
experiencing problems with thinking, understanding, or
remembering things or who sometimes have physical
problems or behavioral changes was conducted from
November 20, 2023, through December 20, 2023. The
sample included White (n=629), Hispanic (n=309), Black
(n=308), Asian (n=206) and Native American (n=24)
caregivers and caregivers who identified as belonging to
other ethnic or racial groups (n=57). While Native
Americans were oversampled in an attempt to get
subgroup estimates, the sample size was still insufficient;
thus, Native American respondents were included in the “all
caregivers” grouping. Respondents were recruited via
non-probability online panels used exclusively for research,
with full population screening data weighted to match U.S.
Census data on age, gender, income, education and race/
ethnicity to ensure accurate representation of the
caregiving population and to establish weighting
benchmarks for demographic oversamples. The survey was
offered in both English and Spanish. Differences noted in
the report between racial and ethnic groups were tested
and found to be statistically significant at the p<.05 level.
Non-Physician Health Care Workforce Survey
A survey of 1,204 U.S. health care workers was conducted
from November 13, 2023, through December 6, 2023.
The survey collected the views of medically-trained and
nonmedically-trained professionals who perform navigation
duties, regardless of whether they describe themselves as
navigators or hold a formal navigator position at their
organization.
A17
For brevity, medically-trained professionals
are referred to as “medical professionals” and nonmedically-
trained professionals as “nonmedical professionals”
throughout the remainder of the report. The report refers
to the combined group of medical and nonmedical
professionals as “health care workers.”
Medical professionals (n=708) included:
• Registered nurses (RN, n=526).
• Nurse practitioners (NP, n=145).
• Physician assistants (PA, n=46).
Nonmedical professionals (n=503) included:
• Social workers (MSW, n=458).
• Community health workers (CHW, n=32).
• Home health aides (HHA, n=14).
Health care workers are classified as both medical and
nonmedical professionals if they indicate both types of
training (e.g., RN with MSW degree). Because of this,
the total of the numbers of medical and nonmedical
professionals shown above exceeds 1,204. Likewise, if
health care workers are classified as having more
than one role in the medical or nonmedical category
(e.g., community health worker and home health aide),
they are included in the count for each role. As a result, the
total of the specific roles in the medical and nonmedical
categories exceeds the 708 and 503 shown above.

99
Dementia Caregiver Survey Results
Dementia Caregiving is a Demanding Job That
Can Last for Years
People with memory and thinking problems see an average
of four different doctors every year, with more than 1 in 4
(27%) seeing five or more doctors annually. Scheduling and
managing doctor's visits can be time-consuming, and more
than 1 in 3 dementia caregivers (35%) coordinate health
care needs (communicating with doctors, taking care of
insurance, getting appointments, picking up medication,
etc.) at least once daily, with some caregivers saying they
coordinated care several times per day.
Caregivers for people with Alzheimer's and other dementia
provide approximately 26 hours of care per week. This is
consistent with other reports that caregivers spend almost
31 hours per week on caregiving.
A8
Additionally, a large
majority of dementia caregivers surveyed spend years
providing care, with nearly 1 in 2 acting as a caregiver for
one to three years and almost 1 in 3 spending four years
or more as a caregiver.
Black Caregivers Report More Time on Caregiving
Responsibilities Than Other Groups
The need to coordinate health care is common for
dementia caregivers, and this is especially true for Black
and Hispanic caregivers, who are more likely to coordinate
health care at least once per day than White caregivers
(43%, 45%, and 31%, respectively). This likely influences the
overall time spent providing care, with Black caregivers
reporting the most time at 30 hours per week followed
by White caregivers (27 hours), Hispanic caregivers
(25 hours) and Asian caregivers (19 hours).
Dementia Caregivers Experience Difficulty and
Stress Interacting With the Health Care System and
Addressing Their Own Needs
A majority of caregivers surveyed (70%) indicated that
coordinating care is stressful. More than half (53%) said
navigating health care for the person they care for was
difficult. Finding resources and support for their needs is
also a challenge for 2 in 3 caregivers (66%; Figure 18).
Black Caregivers Report Less Stress and Difficulty
With Dementia Care
Black caregivers find coordinating dementia care somewhat
less difficult and stressful than all other groups. Three in 5
Black caregivers (58%) reported that coordinating care
was somewhat or very stressful compared with Hispanic
caregivers (71%), White caregivers (72%) and Asian
caregivers (76%). When asked about difficulty coordinating
health care, Asian caregivers expressed the greatest
challenges, with 7 in 10 (68%) indicating that they found it
somewhat or very difficult (vs. Black caregivers, 37%; White
caregivers, 54%; and Hispanic caregivers, 57%). Additionally,
Black caregivers have less difficulty finding support for their
own needs as a caregiver than other groups (52% report
somewhat or very difficult vs. White caregivers, 66%;
Hispanic caregivers, 70%; and Asian caregivers, 77%).
Figure
18
60%
40%
20%
0
Stress of Coordinating Health Care*
Difficulties and Stressors That Caregivers Experience
Very
stressful
Not too
stressful
Somewhat
stressful
Not at all
stressful
52%
24%
5%
18%
70% Stressful 30% Not Stressful
Very
difficult
Somewhat
easy
Somewhat
difficult
Very
easy
53% Difficult 47% Easy
Very
difficult
Somewhat
easy
Somewhat
difficult
Very
easy
66% Difficult 34% Easy
23%
28%
42%
7%
Difficulty of Finding Caregiver Support*
9%
44%
40%
7%
Difficulty of Navigating the
Health Care System
*Percentages of bars may not total the percentages above due to rounding.
Special Report - Mapping a Better Future for Dementia Care Navigation

100 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Worries About Costs and Coordinating Health Care
Are Top Stressors
The most often cited worry for dementia caregivers is cost
(42% of caregivers), followed by the stress of coordinating
with multiple doctors (36%), securing appointments (35%)
and getting help taking a break (35%; Figure 19). Finding
appropriate doctors (32%) rounded out the top five
stressors. When viewed together, these top five stressors
underscore challenges in coordinating dementia health
care without greater assistance from a care navigator.
Asian caregivers report worries about costs and expenses
(51%) as the top stressor, more so than other groups
(Black caregivers, 37%; Hispanic caregivers, 42%; White
caregivers, 41%). Asian caregivers also are more likely to
report stress in finding appropriate doctors (41%) and
understanding recommended treatments (30%) than other
groups. Finding respite care is the top stressor for Black
caregivers (39%), and concerns about cost and expenses
are top of mind for Hispanic caregivers.
Despite these current stressors, only half of the dementia
caregivers surveyed (51%) have ever talked with a health
care professional about challenges finding their way
through the health care system or asked for help with
dementia care.
Although Largely Unfamiliar With the Term
“Care Navigator,” Dementia Caregivers Receive Help
Navigating Care
Three in 4 dementia caregivers surveyed report little or no
familiarity with the term “care navigator,” with 30% saying
they know very little about the term and 45% reporting
they have never heard of the term. Yet half of caregivers
(50%) receive help with dementia health care, support and
services for the care recipient from someone within their
physician's office or hospital. These health care workers
may or may not be serving in a formalized navigator role.
Nurses (42%) or social workers (35%) most often provide
navigation help to dementia caregivers, with physician
assistants (18%), community health workers (14%), other
caregivers (12%) or actual care navigators (7%) providing
health care guidance to a lesser degree (Figure 20).
Nearly 7 in 10 Black caregivers (68% ) report receiving help
navigating care. Asian caregivers (52%), Hispanic caregivers
(51%), and White caregivers (47%) also report currently
receiving help navigating care.
Figure
19
Worries about cost and expenses
Coordinating with multiple doctors
Scheduling and helping with appointments
Getting help to take a break
Finding appropriate doctors
Finding support services and resources
Feeling unprepared to be a caregiver
Transportation to doctor visits
Getting care for urgent needs
Contacting administrators or billing
Getting prescriptions
Understanding recommended treatments
Lack of knowledge about the disease
Finding clinical trials
Stressors When Getting Health Care for Care Recipients
36%
35%
35%
32%
30%
29%
24%
22%
22%
22%
20%
18%
6%
42%

101
When working with staff helping with care navigation, a
majority of caregivers would prefer that they communicate
via more traditional channels, with nearly 2 in 3 saying
a phone call was best and 1 in 2 saying they desired
in-person communication during a visit (Figure 21).
Overall, very few caregivers wanted to communicate
through electronic health record (EHR) messaging or
a patient portal (12%).
Navigator’s Professional Role
Nurse
42%
Social
worker
35%
Physician
assistant
18%
Community
health worker
14%
Caregiver
12%
Navigator
7%
Not sure
1%
Other
4%
50%
40%
30%
20%
10%
0
In-person
visits
Electronic health
record messaging or
patient portal
EmailText
messaging
Video
conferencing
Phone calls
Preferred Channels for Communicating With a Care Navigator
61%
49%
44%
40%
12%
28%
70%
60%
50%
40%
30%
20%
10%
0
Figure
20
Figure
21
Special Report - Mapping a Better Future for Dementia Care Navigation

102 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Caregivers for people living with dementia stressed that it is
essential for care navigators to understand their ethnic or racial
background. Significantly, 9 in 10 Asian, Black, and Hispanic
caregivers felt it crucial for navigators to be aware of the
background of the person they are caring for (Figure 22).
In contrast, White caregivers placed less importance on this
shared experience. Among White caregivers who received
important for navigators to understand (Figure 23). Four in 5
health care workers believe their organization has an effective
understanding of the racial, ethnic, and cultural backgrounds
and experiences of people with dementia and their caregivers.
Cultural Competency is Fundamental for Dementia Care Navigation
navigation help, 84% believed the person helping them had a
good or excellent grasp of their care recipient’s background,
a confidence level higher than that for other racial and ethnic
groups. Confidence in the cultural competency of the person
providing navigation assistance was lower for all other groups, and
lowest for Asian caregivers, with only 54% rating understanding
of the person helping them as “good” or “excellent.”
100%
80%
60%
40%
20%
0
88%
54%
Asian
84%
74%
HispanicBlack
88%
68%
White
57%
84%
Important for care navigator to understand the ethnic or racial background of the person being cared for
Provider of navigator-type service rated “good” or “excellent” on understanding the ethnic or racial background of the person being cared for
Access to Care Navigators With Cultural Competency by Race/Ethnicity of Care Recipient
Health care workers surveyed echoed that empathy for racial,
ethnic and cultural backgrounds and experiences is key. They
overwhelmingly agreed that effective dementia care navigation
requires cultural competence, with 99% saying that it is
Very
important
82%
Somewhat
important
17%
Not too
important
1%
Not at all
important
<1%
99% Important 1% Not Important 81% Effective 19% Not Effective
Very
effective
24%
Somewhat
effective
57%
Not too
effective
17%
Not at all
effective
2%
Health Care Professionals’ Views on Cultural Competence
Importance of Navigators Understanding the
Cultural, Racial and Ethnic Backgrounds and
Experiences of Dementia Patients and Caregivers
Effectiveness of Organization in Understanding the
Cultural, Racial and Ethnic Backgrounds and
Experiences of Dementia Patients and Caregivers
100%
80%
60%
40%
20%
0
Figure
22
Figure
23

103
Caregivers Welcome Dementia Navigation Support
Overwhelmingly, dementia caregivers surveyed would
welcome navigator support, with 4 in 5 caregivers (85%)
indicating that having access to a care navigator would
influence their choice of a dementia health care provider
for the person they care for. This sentiment was strongest
among Black caregivers, with 52% saying it would influence
their choice a great deal, followed by Hispanic caregivers
(44%), White caregivers (43%) and Asian caregivers (38%).
Less Stress and Better Outcomes are Biggest
Benefits of Working With a Dementia Care Navigator
Caregivers see improvement in quality of life (61%) and
health (43%) for the person they care for as being the
greatest positives of working with a dementia care
navigator (Figure 24). Other benefits for the person living
with Alzheimer’s or other dementia include less depression
(35%), longer period of time at home (35%) and fewer
behavioral symptoms (26%).
For themselves, 2 in 5 caregivers surveyed see less stress
(62%) and more peace of mind (62%) as the most valuable
outcomes of having a navigator as part of the dementia
care team (Figure 25). They also think a care navigator
could help them be better caregivers (56%), improve their
mental health (45%) and help them find opportunities to
take a break from their care responsibilities (38%). Overall,
very few dementia caregivers believed a care navigator
would make caregiving less expensive (18%); however,
there was a clear difference in this view among caregivers
from different racial and ethnic groups. Nearly twice as
many Asian, Black and Hispanic caregivers thought working
with a navigator could make caregiving less expensive
compared with White caregivers (26%, 23% and 22%,
respectively, vs. 14%)
Around-the-Clock Support, Care Coordination and
Help Understanding the Care Recipient’s Condition
Viewed as Most Valuable Navigation Services Overall
Nearly all dementia caregivers (97%) say they would find
navigation services helpful. Almost 2 in 5 caregivers (36%)
said a 24/7 helpline to call is the top service a care
navigation program should provide (Figure 26).
Coordinating care and communication between different
specialists (34%) and helping to understand their care
recipient’s condition (34%) are also viewed as valuable
services. Overall, caregivers see value in a mix of medical
and nonmedical navigation services. Other helpful services,
according to 1 in 3 caregivers, included:
• Assistance with insurance or public benefits (32%).
• Help with scheduling appointments (31%).
• Training on how to care for someone with thinking or
memory problems (31%).
• Help managing behavioral symptoms (31%).
• Help understanding the health care system (30%).
• Help finding services to take a temporary break from
caregiving (30%).
Anticipated Outcomes for Care Recipients of a Navigator Program
70%
60%
50%
40%
30%
20%
10%
0
Improvement
in quality of
daily living
61%
Less
depression
35%
43%
Overall
improvement
in health
35%
Longer period
of time living
at home
22%
Fewer
emergency
room visits
21%
Fewer
hospitalizations
14%
Fewer
illnesses
9%
Not sure
26%
Fewer behavioral
symptoms (aggression,
anger, etc.)
2%
None
Figure
24
Special Report - Mapping a Better Future for Dementia Care Navigation

104 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
The findings highlight services more relevant to dementia
care navigation than care navigation for other health
conditions, such as respite services, managing behavioral
symptoms and support for unanticipated needs outside of
regular clinic hours in the form of the helpline. Typically,
community-based organizations provide these services.
Although 1 in 3 caregivers (30%) have received nonmedical
supports and services, such as meal delivery, home care
aides, or companions to give time away from caregiving,
only 17% found these resources with the help of a person
providing care navigation services.
Community-Based Resources May Address
Some Stressors
Community-based resources and services can play an
important role in supporting caregivers. While many of
these services are delivered outside traditional health care
settings, finding ways to connect caregivers to these
resources should be viewed as an essential deliverable in
dementia care navigation.
The three most helpful community-based resources
cited by caregivers in the survey were local caregiver
support groups (41%), respite programs (38%) and
availability of financial resources in the community (37%).
These resources could alleviate some of the top stressors,
like worries about costs and expenses and getting help
taking a break (Figure 27).
Caregivers of all races and ethnicities surveyed pointed to
connections to local adult daycare programs as a valuable
navigation service (Asian, 45%; Black, 36%; Hispanic, 35%;
White, 28%).
Non-Physician Health Care Workforce
Survey Results
“Care Navigator” is a Known Term in Health Care
Three in 4 health care workers who provide navigator-type
services (77%) are familiar with the term “care navigator.”
Nonmedical professionals (CHW, HHA or MSW) were the
most familiar, with 4 in 5 (83%) indicating they had heard
this term before.
Many Professionals Already Provide Navigation
Services, but This is not Their Focus
Health care workers participating in the survey spend
roughly half (53%) their time providing navigator-type
services, even if they do not refer to themselves as care
navigators (Figure 28, page 106). Nearly 1 in 3 of the
patients they provide navigation services for have cognitive
issues, including MCI, Alzheimer’s disease or other
dementia (Figure 28, page 106). The vast majority of
respondents (93%) say that caregivers or family are
almost always involved in discussions of navigation-type
services — with or without the person they are caring for.
Anticipated Outcomes for Caregivers of a Navigator Program
Reducing
the stress of
caregiving
62%
Helping me
be a better
caregiver
56%
Not sure
3%
70%
60%
50%
40%
30%
20%
10%
0
62%
Giving me
more peace
of mind
45%
Improving
my mental
health
Making
caregiving less
time-consuming
34%
18%
Making
caregiving less
expensive
2%
None
38%
Allowing me to
take a break from
caregiving
25%
Improving my
physical health
Figure
25

105
Having a 24/7 support or help line to call
Coordinating care and communication between different specialists
Help understanding the care recipient's condition
Assistance with insurance or public benefits
Help with scheduling appointments
Training on how to care for someone with thinking or memory problems
Help managing behavioral symptoms
Help understanding the health care system
Help finding services to take a temporary break from caregiving
Helping with emotional and cultural support
Help monitoring medications
Assessing whether a medical care plan is on track
Referrals to community support services and resources
Arranging transportation or meal delivery
Help contacting health care administrators or billing
Screening for safety needs
Help with planning for end-of-life decisions
Care Navigator Services That Would Be Valuable to Dementia Caregivers
34%
34%
32%
31%
31%
31%
30%
30%
29%
28%
27%
27%
26%
23%
22%
21%
36%
Community-Based Services That Would Be Valuable to Dementia Caregivers
Local
caregiver
support
groups
41%
Local respite
programs that
provide temporary
care to give
caregivers a break
38%
Financial
resources
in your
community
37%
Local
programs,
workshops and
classes
35%
Disease
information
including a
24/7 helpline
26%
Legal help,
including elder
law attorneys
23%
Local adult
day-care
programs
32%
50%
40%
30%
20%
10%
0
Figure
26
Figure
27
Special Report - Mapping a Better Future for Dementia Care Navigation
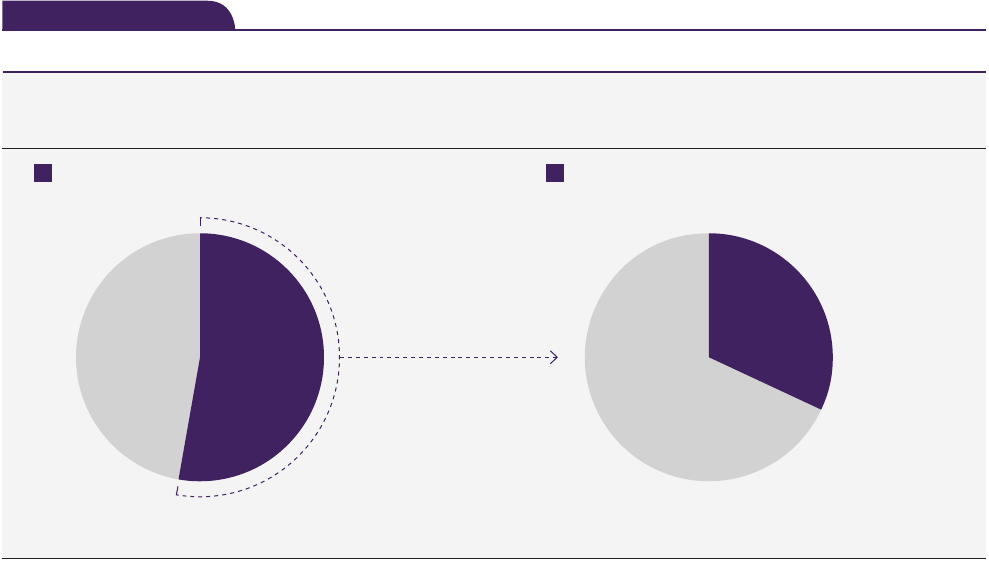
106 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Nearly all health care workers in the survey provide
support for medical and nonmedical needs, with only 5%
focusing exclusively on one type of need (Figure 29). The
most frequently provided navigator services are referrals
to community support services and resources (75%),
helping with emotional and cultural support (68%) and
screening for safety needs (66%) (Figure 30). Of those top
navigation services, referrals to community support are
more often provided by nonmedical professionals, whereas
screening for safety needs is most often performed by
medical professionals.
Unsurprisingly, medical professionals tend to offer more
medically-related navigation services, such as screening for
safety, assessing if the medical plan is on track, monitoring
medications and staffing helplines (Figure 30). They also
viewed these services as more valuable to patients and
families than nonmedical professionals did. Nonmedical
professionals, on the other hand, report that they are more
often involved in making referrals to community resources,
disease education, assisting caregivers looking for respite
care, arranging transportation or meal delivery and
insurance-related support such as working with billing or
insurers (Figure 30).
Further illustrating the complicated nature of dementia
care and the need for navigation as outlined earlier in the
Special Report, health care workers also report that they
are heavily involved in guiding patients and families
through the health care system. Nearly 2 in 3 health care
workers (62%; predominantly nonmedical professionals)
help patients and caregivers understand the health care
system, and more than 1 in 2 health care workers say
they coordinate care and communication with specialists
(Figure 30).
Greatest Value From Navigators is in Connections to
Community Support and Services
More than 2 in 3 health care workers (68%) said the top
service provided, referrals to community support services
and resources, was the most valuable navigation offering
(Figure 31). The top five most valuable navigation services
according to survey respondents were:
• Referrals to community support services and
resources (68%).
• Training on how to care for someone with
dementia (63%).
• Help managing behavioral symptoms (62%).
• Helping with emotional and cultural support (59%).
• Coordinating care and communication between
different specialists (59%).
Time Spent on Navigator-Type Services and Percentage of Patients with Cognitive Issues Receiving Navigation Services
Professional Time Spent Providing
Navigator-Type Services
Patients Receiving Navigation Services
Who Have Mild Cognitive Impairment,
Alzheimer’s Disease or Other Dementia
Mean percentage of time
Mean percentage of patients
53%
32%
Figure
28

107
More than half of respondents also said that finding
respite services to take a temporary break from caregiving,
understanding the health care system, and screening for
safety needs were valuable.
Interestingly, there were two notable disconnects
between what health care workers perceive as valuable
and what they deliver in the form of navigation services.
The first is training for family members on how to care for
someone with dementia. Whereas 63% of survey
respondents rated this as valuable, only 2 in 5 (40%)
provide such training. The second disconnect was in the
utility of a 24/7 helpline. This feature of navigation was
valued by 1 in 3 health care workers (33%), yet only 15%
are currently providing this service.
70%
60%
50%
40%
30%
20%
10%
0
83% Medical 79% Nonmedical
Only
medical
needs
Both
medical and
nonmedical
needs
Mostly
medical
needs
Mostly
nonmedical
needs
Only
nonmedical
needs
2%
19%
62%
14%
3%
Focus of Dementia Care Support Provided
Figure
29
Figure
30
Referrals to community support services and resources
Helping with emotional and cultural support
Screening for safety needs
Help understanding the health care system
Help managing behavioral symptoms
Coordinating care and communication between different specialists
Help understanding mild cognitive impairment or dementia
Help monitoring medications
Help with planning for end-of-life decisions
Assessing whether a medical care plan is on track
Help finding services to take a temporary break from caregiving
Arranging transportation or meal delivery
Assistance with insurance or public benefits
Training on how to care for someone with dementia
Help contacting health care administrators or billing
Providing support via a 24/7 help line
Services Provided That Support Dementia Care for Patients and Their Families
68%
66%
62%
60%
57%
53%
52%
50%
44%
43%
41%
41%
40%
25%
15%
75%
Provided especially by medical professionals Provided especially by nonmedical professionals
Special Report - Mapping a Better Future for Dementia Care Navigation

108 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Nonmedical Professionals Communicate More
Frequently With Patients and Families
Two in 5 nonmedical professionals (39%) report they
connect with individuals living with dementia and their
families every two weeks, and 1 in 3 (32%) make contact
every month. Medical professionals typically communicate
with patients every two weeks (28%) and every month
(24%). However, medical professionals report that they are
more likely to communicate every 3 months and 6 months
than nonmedical professionals (15% vs. 7% and 5% vs. 1%).
This distinction between medical and nonmedical
professionals may be a result of the cadence of follow-up
visits with a physician, and medical professionals may be
more likely to incorporate navigation services into a routine
visit. Across groups, 1 in 4 health care workers (23%)
discuss dementia care services only as needed.
On average, nonmedical professionals interact most often
with patients and families:
• All health care workers: 12 times per year.
• All medical professionals (NP, PA or RN): 11 times
per year.
• All nonmedical professionals (CHW, HHA or MSW):
14 times per year.
Health Care Workers Providing Navigation-Type
Services Use Traditional Communication Channels
Instead of Newer Technologies
In-person visits and phone calls are by far the most
common channels for dementia care navigation and
dramatically outpace usage of newer technologies like video
conferencing, email, patient portals and text messaging.
Care navigation services are 3 to 4 times more likely to be
provided in-person or by phone than other channels, and
most communication still occurs at in-person visits.
There are some distinctions in how medical and nonmedical
professionals deliver navigation services. Medical
professionals communicate more often via a patient portal
than nonmedical professionals (19% vs. 14%). The most
striking difference is how nonmedical professionals appear
to have gravitated toward digital communication, possibly
because they engage with patients and families more
regularly. Nonmedical professionals use video conferencing
and email to reach patients and families twice as often
as medical professionals (29% vs. 11% and 26% vs. 12%,
respectively). Nonmedical professionals also report using
a phone call to communicate more often than medical
professionals (76% vs. 62%).
Figure
31
Referrals to community support services and resources
Training on how to care for someone with dementia
Help managing behavioral symptoms
Helping with emotional and cultural support
Coordinating care and communication between different specialists
Help finding services to take a temporary break from caregiving
Help understanding the health care system
Screening for safety needs
Help understanding mild cognitive impairment or dementia
Help monitoring medications
Help with planning for end-of-life decisions
Assistance with insurance or public benefits
Assessing whether a medical care plan is on track
Arranging transportation or meal delivery
Providing support via a 24/7 help line
Help contacting health care administrators or billing
Most Valuable Services in Supporting Dementia Care for Patients and Their Families
63%
62%
59%
59%
57%
57%
54%
50%
50%
49%
47%
37%
36%
33%
24%
68%
Seen as especially important by medical professionals

109
Training in Dementia Care Navigation is Lacking and
Not Standardized
Three in 4 health care workers providing navigation services
indicated that they received no formal training in dementia
care navigation. The 1 in 4 health care workers who did
receive some kind of training were predominantly
nonmedical professionals and received a median of 30 hours
of formal training. On the other hand, medical professionals
received a median of 20 hours of formal training.
If they received formal training, more than 1 in 2 surveyed
received it from their employer (59%), not from colleges,
universities or other outside programs, such as a certificate
or public health program. Except for nonmedical professionals
being more likely than medical professionals to have exposure
to navigation training during college or university coursework
(47% vs. 23%), there were no differences between the
groups surveyed.
Dementia Care is Rarely the Sole Focus of
Navigation Activities, but Health Care Workers Still
Feel Knowledgeable
Four in 5 health care workers (80%) have navigation
experience in non-dementia medical specialties, and fewer
than 1 in 10 (7%) focus primarily on providing navigator
support and services to people living with dementia. Most
providing navigation services (93%) feel at least somewhat
knowledgeable about MCI, Alzheimer’s disease and other
dementia but only about 1 in 3 (36%) report they are
very knowledgeable.
Central to effective, valuable navigation that benefits
patients and families is a strong knowledge of dementia
care support and resources in health care settings and the
community. Nearly 9 in 10 health care workers (86%) feel
knowledgeable about directing dementia patients and
caregivers to appropriate health care resources, but fewer
than 1 in 3 (30%) report feeling very knowledgeable. Four
in 5 (82%) feel knowledgeable about directing dementia
patients and caregivers to community resources, but only
31% say they are very knowledgeable.
When the group is separated into medical or nonmedical
professionals, nonmedical professionals report feeling
better equipped and more knowledgeable than medical
professionals about health care resources (93% vs. 81%) and
community-based resources (92% vs. 75%). Understandably,
nonmedical professionals feel more capable, given that
they are more likely to communicate regularly with
patients and caregivers and could have received formal
training on delivering navigation services. Additionally,
some health care workers categorized as nonmedical
professionals in this survey, such as community health
workers or home health aides, may have direct exposure or
interaction with resources in the community.
A crucial component of dementia care today is familiarity
with new and emerging treatment options and awareness
of clinical trials. Nearly all health care workers surveyed
believe it is important to be familiar with new treatments
(98%) and with clinical trial options (93%).
Figure
32
82% Effective 18% Not Effective
61%
Very
effective
Not too
effective
Somewhat
effective
Not at all
effective
5%
35%
70%
60%
50%
40%
30%
20%
10%
0
50%
10%
40% Effective 60% Not Effective
Health Care System’s Effectiveness at Helping
Dementia Patients and Families Navigate Health Care
Very
effective
Not too
effective
Somewhat
effective
Not at all
effective
21%
16%
2%
Own Organization’s Effectiveness at Helping
Dementia Patients and Families Navigate Health Care
Health Care Workers’ Views on the Effectiveness of Dementia Care Navigation
Special Report - Mapping a Better Future for Dementia Care Navigation

110 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
The Health Care System Could Do More to Help
People Navigate Dementia Care
Health care workers shed light on current deficits in
dementia care. Sixty percent believe that the U.S. health
care system is not effectively helping patients and their
families navigate dementia care (Figure 32). They perceive
their own organization’s efforts more positively, however,
with 4 in 5 saying that their organization is effective
(somewhat effective [61%] or very effective [21%]) at
providing dementia care; Figure 32). Nearly half surveyed
(46%) say their organizations do not have a clearly defined
process for care coordination and clinical pathways for
patients with MCI, Alzheimer’s disease or other dementia.
Health care workers pointed to a lack of community-based
resources for dementia caregivers and current payment
models that do not incentivize care coordination as the
greatest barriers to dementia care navigation (Figure 33).
More than 3 in 4 of the health care workers surveyed (77%)
identified a lack of community-based resources as a barrier,
and 44% viewed it as the greatest barrier. Seven in 10 (70%)
called out restrictions in current reimbursement as a
barrier, with 41% saying this was the greatest barrier.
Interestingly, health care workers did not identify
workforce shortages as a top limitation for dementia care
navigation (Figure 33). There were no differences in
perceived barriers to dementia care navigation between
medical and nonmedical professionals.
Almost 9 in 10 health care workers (87%) feel that
developing alternative payment models is important in
providing future care coordination for people diagnosed
with dementia.
Nonmedical Professionals Are Best Suited to be
Dementia Care Navigators
Survey respondents overwhelmingly agreed that
nonmedical professionals are best suited to provide
navigation services (Figure 34). Within their
organizations, those surveyed reported that social
workers, community health workers, and home health
aides are more often formally involved in care navigation,
and 9 in 10 reported that these individuals are best suited
to offer navigation services. The next group of individuals
health care workers believed are suited for navigation
roles are former caregivers or others who have lived the
caregiving experience, but few reported that these
individuals are formally involved in dementia care
navigation at their organizations. Surprisingly, nearly
2 in 3 health care workers indicated that physicians at
their organization are involved in helping patients and
caregivers navigate health care but fewer than half of the
health care workers surveyed think that physicians are
best suited for this work.
Figure
33
77%
50%
44%
10%
Lack of community-
based resources for
dementia caregiver
Lack of trained health
care social workers
Current payment
models do not incentivize
care coordination
Lack of trained nurse
practitioners
70%
29%
41%
3%
Seen as a barrier Seen as the biggest barrier
Barriers to Dementia Care Navigation
80%
60%
40%
20%
0

111
A Path Forward: Revolutionizing Dementia
Care With Person-Centered Navigation
As the complexity of health care for Alzheimer’s and
other dementias continues to challenge individuals living
with dementia and caregivers alike, the Alzheimer’s
Association dementia caregiver and non-physician health
care workforce surveys call attention to the urgent
need for person-centered dementia care navigation and
care delivery solutions for health systems, health care
professionals, caregivers and people living with dementia.
Dementia care management is emerging as an ideal model
to unravel dementia care complexity, improve outcomes
and lower costs (Figure 35).
978
Care navigation is a crucial
component that touches all other aspects of care
management, such as caregiver education and training,
care coordination, medication management, management
of chronic conditions, safety assessments, and advance
care planning.
978
Dementia care navigation, as part of
comprehensive dementia care management, has the
potential to revolutionize care if it is:
• Person-centered to meet the evolving, unique needs of
all individuals living with Alzheimer’s or other dementia.
• Durable yet adaptable to accommodate new treatments,
new diagnostics and other improvements to care.
• Comprehensive to cover medical and nonmedical needs.
• Coordinated to connect disparate care teams and
community resources.
• Feasible regardless of health system structure.
• Cognizant of geographic and socioeconomic barriers.
Ultimately, the goal of care navigation is to improve the
quality of life for people living with dementia, reduce
caregiver stress and enable people living with dementia to
live in their homes and communities as long as possible.
The GUIDE Model is a reason for optimism that
emphasizes streamlined care coordination and robust
support for caregivers — including forging a vital
connection with a dedicated care navigator — and
creating an alternative payment model to reimburse
physicians (see What is GUIDE? on page 94).
986
However, while the GUIDE Model offers one potential
approach toward enhancing dementia care navigation,
it is a limited pilot program that will not be delivered by
all health care providers nor available to all patients with
dementia. Therefore, it is important for health systems,
private insurers and other stakeholders to develop their
own strategies to help people living with dementia and
their caregivers navigate care.
The Special Report survey results brought to light three
themes to advance dementia care navigation efforts:
1.
Formalizing the dementia care navigator role and
increasing navigator proficiency in dementia care.
2.
Scaling and expanding access to dementia care
navigation programs.
3.
Creating direct lines to dementia care navigators.
Special Report - Mapping a Better Future for Dementia Care Navigation
Figure
34
85%
56%
92%
44%
Social workers,
community health workers,
home health aides
Nurse practitioners,
physician assistants,
registered nurses
Physicians Former caregivers of
others who have lived
the dementia caregiving
experience
61%
18%
40%
46%
Who is formally involved Who is best suited
Professionals Who Help Dementia Patients and Caregivers Navigate Health Care
100%
80%
60%
40%
20%
0

112 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
Formalize the Dementia Care Navigator Role
Survey responses revealed a noteworthy trend: many
health care workers are undertaking navigator duties
in addition to their usual responsibilities. Ideally, practices
and health systems should transition from ad hoc
navigation support to formalizing dedicated dementia
care navigator roles that are recognized for their vital
and essential contributions to interdisciplinary, person-
centered dementia care.
As mentioned in the Workforce section (see page 64),
several decades of research support the value of
collaborative models that bring different health
professionals together, such as social workers, registered
nurses and non-clinical care managers, physicians and
advanced practice providers, and direct care workers,
including nurse aides, nursing assistants, home health
aides and personal care aides.
754, 755
If these individuals are
the spokes in the dementia care wheel, the care navigator
is the hub — supporting patients and caregivers as they
find their way through the dementia care ecosystem.
Given that this is a relatively new role in the dementia
workforce, it’s crucial for organizations to create a
practice framework to seamlessly integrate dementia
care navigators into existing teams and workflows to
effectively coordinate longitudinal care that spans the
disease course.
Increase Dementia Proficiency to Cultivate
Specialist Navigators
In identifying ideal candidates for navigator roles, medical
and nonmedical professionals agreed that community
health workers, social workers and home health aides are
best suited to be dementia care navigators. Despite
receiving some formal education in navigation, often
The Ideal Future State of Dementia Care
978
as part of college coursework or employer-provided
training, these professionals are typically trained as
generalists. Supplemental training and resources are
necessary to build a solid foundation in both the practical
and emotional aspects of dementia care. Their skills
and compassion for the challenges caregivers and
individuals living with dementia face could be enhanced
with supplemental dementia-specific training and
resources, such as:
• Materials for health care workers from the Health
Resources & Services Administration.
996
• Professional development programs in dementia care
recognized by the Alzheimer’s Association.
997
• The Care Ecosystem Toolkit from the University
of California, San Francisco (UCSF) Memory and
Aging Center.
998
• Home health clinician manuals from the Wisconsin
Alzheimer’s Institute.
999
• Caregiver training videos from UCLA Health.
1000
• Resources, webinars, presentations and toolkits
from the National Alzheimer’s and Dementia
Resource Center.
1001
• Community health worker training for participants
in programs funded by the Administration for
Community Living-Alzheimer’s Disease Program
Initiative (ACL-ADPI).
982
• Resources and webinars on brain health and
dementia from the National Association of
Community Health Centers.
1002
To bolster the training and resources outlined here, the
Alzheimer’s Association is developing a person-centered
navigator training curriculum and certification that is
slated for release in late 2024. This curriculum, which
Patient
+
Caregiver
Dementia Care
Management
(Including
Navigation)
Specialist
Social Services
Medication
Caregiver Supports
Primary Care Provider
Better
Dementia Care
Improved
Outcomes for
Patients and
Caregivers
Loer
Medicare
Costs
Figure
35
113
incorporates the Alzheimer’s Association's evidence-
based Dementia Care Practice Recommendations, has the
potential to increase the proficiency of care navigators,
ensuring that they are well-equipped to meet the distinct
needs of individuals living with dementia and their
caregivers. Investment in navigator training and
development could yield a marked improvement in the
overall quality and effectiveness of dementia care.
Incentivize Scalability of Dementia Care Navigation
to Expand Reach
Fee-for-service payment has dominated the health care
market. Under these structures, health care providers are
paid for individual services they perform, such as office
visits or tests.
1003
Experts have long argued that fee-for-
service is inefficient because it encourages the delivery
of more potentially unnecessary care while discouraging
care coordination.
1004
The U.S. health care system is
increasingly transitioning from fee-for-service structures
to alternative payment models, including value-based
payment.
1004
Often called “volume to value,” the goal
of value-based payments is to restructure the approach
“from one that incentivizes volume to one that
rewards value.”
1005
Dementia care is not immune from financial
incentives.
984, 1006, 1007
Health care workers in this year’s
survey believed that current reimbursement systems fail
to incentivize dementia care and are one of the greatest
barriers to dementia care navigation. They strongly
believe that alternative payment models are important in
providing future care coordination for people diagnosed
with dementia.
Work to Make Existing and Future Dementia Care
Navigation Programs Visible and Accessible
According to this year’s caregiver survey, awareness
of dementia care navigators remains low despite the
recognized value of navigation. This may, in part, be due
to variations in terminology used by different health care
providers, inconsistent definitions or that these individuals
simply do not yet exist within organizations. Depending
on the setting, what is defined as dementia care
navigation in this Special Report may also be called
memory care navigation, care navigation, a navigator
program, dementia navigation or not have terminology at
all, but simply be services provided to patients.
990, 992
Future programs must focus on elevating the visibility of
navigation services through targeted outreach efforts.
This involves leveraging community resources, social
media and health care settings to inform and educate
dementia caregivers about the support available to them.
A compendium that defines terms and lists programs by
region could be a useful tool to empower individuals living
with Alzheimer’s or other dementia and their caregivers.
Furthermore, integrating care navigation into primary
care and specialty clinics can ensure that more patients
and families benefit from these services from the onset
of their dementia care journey.
Another key factor limiting access to existing dementia
care navigation programs is their location. Existing
dementia care navigation programs are typically housed
within large health systems or academic medical centers,
putting them out of reach for many individuals from rural
and underrepresented communities who receive care
from hospitals or clinics within their community.
992
In
anticipation of the growing need for dementia care
navigation programming, the Alzheimer’s Association
created the Dementia Care Navigation Roundtable,
which will help organizations establish best practices,
support implementation and increase access to
navigation programs.
Leverage 24/7 Helplines and Technology to Create
Direct Lines to Care Navigators
Dementia caregivers reported that the most valuable
service that care navigation could offer would be a 24/7
helpline. The Alzheimer’s Association currently offers a
24/7 helpline that performs some navigation activities,
such as assisting individuals with Alzheimer’s or other
dementia and their caregivers with recommendations for
finding qualified care providers, general information
about legal, financial and care decisions, and referrals to
local programs and services.
1008
Ideally, access to 24/7
assistance would be connected directly with an
individual’s interdisciplinary care team. This allows the
care team to manage longitudinal care, proactively assess
any changes needed in care and potentially mitigate
unnecessary emergencies.
While dementia caregivers and health care workers
acting as navigators still prefer traditional communication
methods such as phone calls and in-person visits for
everyday communication, there is an opportunity to
integrate technology solutions to streamline care
coordination and support; these solutions should be
viewed as complementary to existing person-centered
approaches rather than replacements. Several companies
are exploring on-demand virtual and app-based dementia
care navigation, and the GUIDE Model supports
contracting with suppliers to meet care delivery
requirements that participants in the model wouldn’t
otherwise be able to meet on their own.
986
Digital
platforms can offer caregivers and patients easier access
to resources, appointment scheduling and direct
communication with care navigators. However, any
technological solution must be user-friendly and
accessible to all caregivers, regardless of their familiarity
Special Report - Mapping a Better Future for Dementia Care Navigation
114 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
with digital tools, and compatible with any platforms
used by health care providers. Navigators should be
trained on how to communicate effectively through
different channels.
Conclusion
The path forward for person-centered dementia care
navigation is illuminated by the insights and experiences
of dementia caregivers and health care professionals.
The first step on this path is to establish proficient,
dedicated dementia care navigators as a new role in the
interdisciplinary dementia care workforce. Then, by
addressing these key areas — training, person-centered
care, accessibility, collaboration, novel payment models,
and technology — future dementia care navigation
programs can build on the learnings of their predecessors.
Such efforts strive to improve the quality of life for
individuals living with dementia and their caregivers and
pave the way for a more sustainable, efficient and
compassionate health care system.

115
A1. Racial and ethnic identifiers: Facts and Figures keeps the racial
and ethnic terms used in source documents when describing
study findings. When not referring to data from specific studies,
adjectives such as “Black,” “Hispanic” and “White” may be used
(for example, Black populations and Hispanic communities).
A2. Estimated prevalence (number and proportion) of Americans age
65 and older with Alzheimer’s dementia for 2024: The estimated
6.9 million individuals ages 65 years and older with Alzheimer’s
dementia and the estimated numbers of individuals with
Alzheimer’s in each age group were reported from a study that
used data from the Chicago Health and Aging Project (CHAP) in
combination with population projections from the U.S. Census.
241
The number, 6.9 million, is higher than estimated from previous
study that also combined CHAP and U.S. Census data. This is
because the more recent study used updated Census projections
and incorporated information from Hispanic/Latino American
individuals. The proportion of the population with Alzheimer’s
dementia (among people age 65 and older and by age group) is
calculated using as the numerators the numbers of people with
Alzheimer’s dementia, as reported by the recent study in
CHAP.
241
The denominators were the U.S. Census population
projections for the specific age groups of interest.
A3. Differences between CHAP and HRS-HCAP estimates for
Alzheimer’s dementia prevalence: The number of people
estimated to have any form of dementia in the U.S. in 2016 from
the Health and Retirement Study’s (HRS) Harmonized Cognitive
Assessment Protocol (HCAP; 4.92 million) is lower than the
CHAP estimate of how many people were living with Alzheimer’s
dementia only (6.07 million).
160
This is because of differences in
dementia ascertainment between the two studies: both studies
used scores on batteries of cognitive tests, but the HRS-HCAP
study additionally required an informant report of functional
impairment (i.e. disability). Because the more stringent threshold
for dementia in HRS-HCAP may miss people with mild
Alzheimer’s dementia, the Association believes that the larger
CHAP estimates may be a more relevant estimate of the burden
of Alzheimer’s dementia in the United States.
A4. Criteria for identifying people with Alzheimer’s or other
dementias in the Framingham Heart Study: From 1975 to 2009,
7,901 people from the Framingham Study who had survived free
of dementia to at least age 45, and 5,937 who had survived free
of dementia until at least age 65 were followed for incidence of
dementia.
286
Diagnosis of dementia was made according to the
Diagnostic and Statistical Manual of Mental Disorders, 4th
Edition (DSM-IV) criteria and required that the participant
survive for at least 6 months after onset of symptoms. Standard
diagnostic criteria (the NINCDS-ADRDA criteria from 1984)
were used to diagnose Alzheimer’s dementia. The definition of
Alzheimer’s and other dementias used in the Framingham Study
was very strict; if a definition that included milder disease and
disease of less than six months’ duration were used, lifetime risks
of Alzheimer’s and other dementias would be higher than those
estimated by this study.
A5. Projected number of people with Alzheimer’s dementia,
2020-2060: This figure comes from the CHAP study.
241
Other projections are somewhat lower (see, for example,
Brookmeyer et al.
1009
) because they relied on more conservative
methods for counting people who currently have Alzheimer’s
dementia.
A3
Nonetheless, these estimates are statistically
consistent with each other, and all projections suggest
substantial growth in the number of people with Alzheimer’s
dementia over the coming decades.
A6. Annual mortality rate due to Alzheimer’s disease by state:
Unadjusted death rates are presented rather than age-adjusted
death rates in order to provide a clearer depiction of the burden
of mortality for each state. States such as Florida with larger
populations of older people will have a larger burden of mortality
due to Alzheimer’s — a burden that appears smaller relative to
other states when the rates are adjusted for age.
A7. Number of family and other unpaid caregivers of people with
Alzheimer’s or other dementias: To calculate this number, the
Alzheimer’s Association started with data from the Behavioral
Risk Factor Surveillance System (BRFSS) survey. Since 2016, all
states and the District of Columbia utilized the BRFSS caregiver
module. This module identified respondents age 18 and over
who had provided any regular care or assistance during the past
month to a family member or friend who had a health problem,
long-term illness or disability. The module asks a series of
follow-up questions, including asking the caregiver to identify
what the main health problem, long-term illness, or disability that
the person they care for has. One of the reported condition
categories is “Alzheimer’s disease, dementia, or other cognitive
impairment.” In the BRFSS surveys conducted in 2019 and after,
an additional follow-up question was included, asking if the
caregiving recipient also had dementia in addition to their main
condition. Prior to 2019, the survey did not include caregivers of
recipients for whom dementia was not their main condition, so
these numbers were imputed using data collected in 2019 by the
National Alliance for Caregiving (NAC)/AARP survey. The NAC/
AARP survey asked respondents age 18 and over whether they
were providing unpaid care for a relative or friend age 18 or
older or had provided such care during the past 12 months.
Respondents who answered affirmatively were then asked about
the health problems of the person for whom they provided care:
11% of respondents reported dementia as the main condition of
their care recipient, while 26% of all respondents reported the
presence of dementia. Using this ratio in combination with
BRFSS data, the Alzheimer’s Association was able to determine
the percentage of adults in all states and the District of Columbia
who are caregivers for individuals living with Alzheimer’s or
another dementia. These percentages were applied to the
estimated number of people age 18 and older in each state
in July 2023, using U.S. Census Bureau data available at:
https://www.census. gov/programs-surveys/popest/data/tables.
html. This resulted in a total of 11.457 million Alzheimer’s
and dementia caregivers across all 50 states and the District
of Columbia.
A8. Number of hours of unpaid care: The BRFSS survey asks
caregivers to identify, within five time frames, the number of
hours they provide care in an average week. Using the method
developed by Rabarison and colleagues,
441
the Alzheimer’s
Association assumed the midpoint of each time frame was the
average number of hours for each caregiver within that time
frame and then calculated the overall average number of hours
of weekly care provided by dementia caregivers in each state.
This number was then converted to a yearly average and
multiplied by the number of caregivers in each state
A7
to
determine the total number of hours of care provided. When
added together, across all 50 states and the District of Columbia,
the total number of hours provided by Alzheimer’s and dementia
caregivers is 18.376 billion hours.
A9. Value of unpaid caregiving: For each state, the hourly value of
care was determined as the average of the state minimum hourly
wage
1010
and the most recently available state median hourly cost
of a home health aide. (For Nevada, the minimum wage used was
the average of the minimum wage for those who are not
provided health insurance and the minimum wage for those who
are provided health insurance.)
908
The average for each state was
then multiplied by the total number of hours of unpaid care in
that state
A8
to derive the total value of unpaid care. Adding the
totals from all states and the District of Columbia resulted in an
economic value of $346.585 billion for dementia caregiving in
the United States in 2023.
Appendices
End Notes
Appendices

116 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
A10. The 2014 Alzheimer’s Association Women and Alzheimer’s Poll:
This poll questioned a nationally representative sample of
3,102 American adults about their attitudes, knowledge and
experiences related to Alzheimer’s and dementia from Jan. 9,
2014, to Jan. 29, 2014. An additional 512 respondents who
provided unpaid help to a relative or friend with Alzheimer’s or
a related dementia were asked questions about their care
provision. Random selections of telephone numbers from
landline and cell phone exchanges throughout the United States
were conducted. One individual per household was selected
from the landline sample, and cell phone respondents were
selected if they were 18 years old or older. Interviews were
administered in English and Spanish. The poll “oversampled”
Hispanics/Latinos, selected from U.S. Census tracts with higher
than an 8% concentration of this group. A list sample of Asian
Americans was also utilized to oversample this group. A general
population weight was used to adjust for number of adults in the
household and telephone usage; the second stage of this weight
balanced the sample to estimated U.S. population characteristics.
A weight for the caregiver sample accounted for the increased
likelihood of female and White respondents in the caregiver
sample. Sampling weights were also created to account for the
use of two supplemental list samples. The resulting interviews
comprise a probability-based, nationally representative sample
of U.S. adults. A caregiver was defined as an adult over age 18
who, in the past 12 months, provided unpaid care to a relative or
friend age 50 or older with Alzheimer’s or another dementia.
Questionnaire design and interviewing were conducted by Abt
SRBI of New York.
A11. Lewin Model on Alzheimer’s and dementia costs: These numbers
come from a model created for the Alzheimer’s Association by
the Lewin Group. The model estimates total payments for health
care, long-term care and hospice — as well as state-by-state
Medicaid spending — for people with Alzheimer’s and other
dementias. The model was updated by the Lewin Group in
January 2015 (updating previous model) and June 2015
(addition of state-by-state Medicaid estimates). Detailed
information on the model, its long-term projections and its
methodology are available at: alz.org/trajectory. For the
purposes of the data presented in this report, the following
parameters of the model were changed relative to the
methodology outlined at alz.org/trajectory: (1) cost data from
the 2018 Medicare Current Beneficiary Survey (MCBS) were
used rather than data from the 2008 MCBS; (2) prevalence
among older adults was assumed to equal the prevalence levels
from Rajan and colleagues
241
and included in this report
(6.9 million in 2024), rather than the prevalence estimates
derived by the model itself; (3) estimates of inflation and excess
cost growth reflect the most recent relevant estimates from the
cited sources (Centers for Medicare & Medicaid Services [CMS]
actuaries and the Congressional Budget Office); and (4) 2014
state-by-state data from CMS on the number of nursing home
residents and percentage with moderate and severe cognitive
impairment were used in lieu of 2012 data. The Lewin Model's
state-specific Medicaid costs for 2020 and 2025 are based on an
earlier estimate of state prevalence than reported here (Weuve
J, Hebert LE, Scherr PA, Evans DA. Prevalence of Alzheimer
disease in U.S. states. Epidemiology 2015;26(1):E4-6).
A12. All cost estimates were inflated to year 2023 dollars using the
Consumer Price Index (CPI): All cost estimates were inflated
using the seasonally adjusted average prices for medical care
services from all urban consumers. The relevant item within
medical care services was used for each cost element. For
example, the medical care item within the CPI was used to inflate
total health care payments; the hospital services item within the
CPI was used to inflate hospital payments; and the nursing home
and adult day services item within the CPI was used to inflate
nursing home payments.
A13. Average annual per-person payments for health care and
long-term care services for Medicare beneficiaries age 65 and
older with and without Alzheimer’s or other dementias:
Payments are unadjusted, and therefore, do not account for
differences in patient characteristics, such as age or sex.
Additionally, payments are based on health care utilization and
payments in 2018, prior to the COVID-19 pandemic, and do not
reflect any pandemic-related changes in utilization.
A14. Enrollment in fee-for-service Medicare versus Medicare Part C:
Individuals eligible for Medicare can enroll in traditional
Medicare, also referred to as fee-for-service Medicare and
original Medicare, or Medicare Advantage, also referred to as
Medicare Part C.
1011
With traditional Medicare, beneficiaries can
receive care from any doctor or hospital that accepts Medicare
in the United States. Generally, beneficiaries can seek care from
a specialist without a referral. Traditional Medicare has fixed cost
sharing, which includes coinsurance of 20% of the Medicare-
approved amount for services covered by Part B after the
deductible is met. Individuals enrolled in traditional Medicare can
also enroll in Medicare Supplemental Insurance (also referred to
as Medigap) to help cover the out-of-pocket costs. Traditional
Medicare does not have an annual limit on the amount
beneficiaries pay out-of-pocket. Benefits are the same for all
individuals enrolled in traditional Medicare. Individuals enrolled in
traditional Medicare can also enroll in a Medicare Part D plan to
cover some of the costs of prescription drugs. Medicare Part D
enrollment has a separate premium. With Medicare Advantage,
individuals must enroll in a specific private plan. Premiums,
benefits and out-of-pocket costs may vary across plans.
Medicare Advantage plans have an annual limit on the amount
individuals pay out-of-pocket. Individuals enrolled in a Medicare
Advantage plan are not allowed to enroll in Medigap. Medicare
Advantage plans are also allowed to offer additional benefits not
included in traditional Medicare, such as vision, hearing and
dental services as well as some non-health care benefits, such as
transportation costs and gym memberships. Many Medicare
Advantage plans include prescription drug coverage (Medicare
Part D). Individuals enrolled in a Medicare Advantage plan have a
specific network of doctors and hospitals that enrollees need to
use for services to be paid by the Medicare Advantage plan.
Additionally, individuals enrolled in a Medicare Advantage plan
may need a referral to see a specialist. Enrollment in Medicare
Advantage has increased dramatically over the past decade, with
51% of all Medicare beneficiaries enrolled in a Medicare
Advantage plan in 2023 compared to 29% in 2013.
918
A15. Medicare Current Beneficiary Survey Report: These data come
from an analysis of findings from the 2018 Medicare Current
Beneficiary Survey (MCBS). The analysis was conducted for the
Alzheimer’s Association by Health Care Cost Institute.
863
The
MCBS, a continuous survey of a nationally representative sample
of about 15,000 Medicare beneficiaries, is linked to Medicare
claims. The survey is supported by the U.S. Centers for Medicare
& Medicaid Services (CMS). For community-dwelling survey
participants, MCBS interviews are conducted in person three
times a year with the Medicare beneficiary or a proxy
respondent if the beneficiary is not able to respond. For survey
participants who are living in a nursing home or another
residential care setting, such as an assisted living residence,
retirement home or a long-term care unit in a hospital or mental
health facility, MCBS interviews are conducted with a staff
member designated by the facility administrator as the most
appropriate to answer the questions. Data from the MCBS
analysis that are included in 2024 Alzheimer’s Disease Facts and
Figures pertain only to Medicare beneficiaries age 65 and older.

117
Appendices
For this MCBS analysis, people with dementia are defined as:
A16. Differences in estimated costs reported by Hurd and colleagues:
Hurd and colleagues
862
estimated per-person costs using data
from participants in ADAMS, a cohort in which all individuals
underwent diagnostic assessments for dementia. One reason
that the per-person costs estimated by Hurd and colleagues are
lower than those reported in Facts and Figures is that ADAMS,
with its diagnostic evaluations of everyone in the study, is more
likely than MCBS to have identified individuals with less severe
or undiagnosed Alzheimer’s. By contrast, the individuals with
Alzheimer’s registered by MCBS are likely to be those with more
severe, and therefore more costly, illness. A second reason is that
the Hurd et al. estimated costs reflect an effort to isolate the
incremental costs associated with Alzheimer’s and other
dementias (those costs attributed only to dementia), while the
per-person costs in 2024 Alzheimer’s Disease Facts and Figures
incorporate all costs of caring for people with the disease
(regardless of whether the expenditure was related to dementia
or a coexisting condition).
A17. For the health care workforce survey, medical and nonmedical
respondents were required to meet the following
screening criteria:
• Community-dwelling survey participants who answered yes
to the MCBS question, “Has a doctor ever told you that you had
Alzheimer’s disease or dementia?” Proxy responses to this
question were accepted.
• Survey participants who were living in a nursing home or other
residential care setting and had a diagnosis of Alzheimer’s
disease or dementia in their medical record
• Survey participants who had at least one Medicare claim with a
diagnostic code for Alzheimer’s or other dementias in 2008.
The claim could be for any Medicare service, including hospital,
skilled nursing facility, outpatient medical care, home health
care,hospice or physician, or other health care provider visit.
The diagnostic codes used to identify survey participants with
Alzheimer’s or other dementias are 331.0, 331.1, 331.11,
331.19, 331.2, 331.7, 331.82, 290.0, 290.1, 290.10, 290.11,
290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41,
290.42, 290.43, 291.2, 294.0, 294.1, 294.10 and 294.11.
Costs from the MCBS analysis are based on responses from
2018 and reported in 2023 dollars.
• Spend at least 20% of professional time interacting
directly with patients or caregivers.
• Report at least 10% of their patients being age 60 or older.
• Spend at least 10% of professional time performing
navigation-type services.
• Report at least 10% of patients for whom they perform
these services having Alzheimer’s disease, other dementia
or mild cognitive impairment (MCI).
Dementia care navigation was defined as the following for
this group:
Whether or not you consider yourself a care navigator, do you
provide any of these types of care, support or services in your
current role?
• Patient education around diagnosis, treatment options
and resources.
• Patient referrals to clinical specialists.
• Patient referrals to clinical trials.
• Patient referrals to social workers.
• Patient referrals to community-based services.
• Patient scheduling (labs, care team appointments, etc.)
• Patient assistance with insurance.
118 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
1. Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA,
Salvado O, et al. Amyloid ß deposition, neurodegeneration, and
cognitive decline in sporadic Alzheimer’s disease: A prospective
cohort study. Lancet Neurol 2013;12(4):357-67.
2. Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardos C,
Jimenez-Del-Rio M, et al. Brain imaging and fluid biomarker
analysis in young adults at genetic risk for autosomal dominant
Alzheimer’s disease in the presenilin 1 E280A kindred: A
case-control study. Lancet Neurol 2012;11(2):1048-56.
3. Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML,
Knopman DS, et al. Serial PiB and MRI in normal, mild cognitive
impairment and Alzheimer’s disease: Implications for sequence
of pathological events in Alzheimer’s disease. Brain
2009;132:1355-65.
4. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox
NC, et al. Clinical and biomarker changes in dominantly
inherited Alzheimer’s disease. N Engl J Med 2012;367(9):795-804.
5. Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S,
et al. Spatial patterns of neuroimaging biomarker change in
individuals from families with autosomal dominant Alzheimer’s
disease: A longitudinal study. Lancet Neurol 2018;17(3):241-50.
6. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the
pathologic process in Alzheimer disease: Age categories from
1 to 100 years. J Neuropathol Exp Neurol 2011;70(11):960-9.
7. Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller
JT, et al. Plasma neurofilament light chain in the presenilin 1
E280A autosomal dominant Alzheimer's disease kindred: A
cross-sectional and longitudinal cohort study. Lancet Neuro
2020;19(6):513-21.
8. Barthelemy N, Joseph-Mathurin N, Gordon BA, Hassenstab,
Benzinger TLS, et al. A soluble phosphorylated tau signature
links tau, amyloid and the evolution of stages of dominantly
inherited Alzheimer’s disease. Nat Med 2020;26:398-407.
9. Byard RW, Langlois NEI. Wandering dementia: A syndrome
with forensic implications. J Forensic Sci 2019;64(2):443-5.
10. Tom SE, Hubbard RA, Crane PK, Haneuse SJ, Bowen J,
McCormick WC, et al. Characterization of dementia and
Alzheimer's disease in an older population: Updated incidence
and life expectancy with and without dementia. Am J Public
Health 2015;105(2):408-13.
11. Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST.
Alzheimer disease and mortality: A 15-year epidemiological
study. Arch Neurol 2005;62(5):779-84.
12. Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W.
Survival among patients with dementia from a large multi-
ethnic population. Alzheimer Dis Assoc Disord 2005;19(4):
178-83.
13. Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival
following a diagnosis of Alzheimer disease. Arch Neurol
2002;59(11):1764-7.
14. Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD,
Teri L, et al. Survival after initial diagnosis of Alzheimer disease.
Ann Intern Med 2004;140(7):501-9.
15. Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N,
Stern Y. Survival in Alzheimer disease: A multiethnic,
population-based study of incident cases. Neurology
2008;71(19):1489-95.
16. Xie J, Brayne C, Matthews FE. Survival times in people with
dementia: Analysis from a population based cohort study with
14-year follow-up. BMJ 2008;336(7638):258-62.
17. Brodaty H, Seeher K, Gibson L. Dementia time to death: A
systematic literature review on survival time and years of life
lost in people with dementia. Int Psychogeriatr
2012;24(7):1034-45.
18. Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia
and predictors of mortality: A review. Int J Geriatr Psychiatry
2013;28(11):1109-24.
19. World Health Organization. Dementia: Key facts. Available at:
https://www.who.int/news-room/fact-sheets/detail/dementia.
Accessed October 10, 2023.
20. Smith AD, Smith SM, de Jager, CA, Whitbread P, Johnston C,
Agacinski G, et al. Homocysteine-lowering by B vitamins slows
the rate of accelerated brain atrophy in mild cognitive
impairment: A randomized controlled trial. Plos One
2010;5(9):e12244.
21. Kapasi A, DeCarli C, Schneider JA. Impact of multiple
pathologies on the threshold for clinically overt dementia. Acta
Neuropathol 2017;134(2):171-86.
22. Brenowitz WD, Hubbard RA, Keene CD, Hawes SE, Longstreth
WT, Woltjer, et al. Mixed neuropathologies and estimated rates
of clinical progression in a large autopsy sample. Alzheimers
Dement. 2017;13(6):654-62.
23. National Institute on Aging. What are frontotemporal
disorders? Available at: https://www.nia.nih.gov/health/
what-are-frontotemporal-disorders. Accessed December 16, 2023.
24. Hogan DB, Jette N, Fiest KM, Roberts JI, Pearson D, Smith EE,
et al. The prevalence and incidence of frontotemporal
dementia: A systematic review. Can J Neurol Sci
2016;43(suppl):S96-109.
25. Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal
sclerosis dementia differs from hippocampal sclerosis in frontal
lobe degeneration. Acta Neuropathol. 2007 Mar;113(3):245-52.
26. Kane JPM, Surendranathan A, Bentley A, Sally A H Barker SAH,
Taylor J-P, Thomas AJ, et al. Clinical prevalence of Lewy body
dementia. Alzheimers Res Ther 2018 Feb 15;10(1):19.
27. De Reuck J, Maurage CA, Deramecourt V, Pasquier F,
Cordonnier C, Leys D, et al. Aging and cerebrovascular lesions
in pure and in mixed neurodegenerative and vascular dementia
brains: A neuropathological study. Folia Neuropathol
2018;56(2):81-7.
28. James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA.
Dementia from Alzheimer disease and mixed pathologies in the
oldest old. JAMA 2012;307(17):1798-1800.
29. Stojkovska I, Krainc D, Mazzulli JR. Molecular mechanisms of
-synuclein and GBA1 in Parkinson's disease. Cell Tissue Res
2018;373(1):51-60.
30. Aarsland D, Zaccai J, Brayne C. A systematic review of
prevalence studies of dementia in Parkinson's disease. Mov
Disord. 2005;20(10):1255.
31. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM.
Multiple pathologies are common and related to dementia in
the oldest-old: The 90+ Study. Neurology 2015;85(6):535-42.
32. Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and
dementia: How to move forward? Neurology 2009;72:368-74.
33. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain
pathologies account for most dementia cases in community-
dwelling older persons. Neurology 2007;69:2197-204.
34. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The
neuropathology of probable Alzheimer disease and mild
cognitive impairment. Ann Neurol 2009;66(2):200-8.
35. Jellinger KA, Attems J. Neuropathological evaluation of mixed
dementia. J Neurol Sci 2007;257(1-2):80-7.
36. Jellinger KA. The enigma of mixed dementia. Alzheimers
Dement 2007;3(1):40-53.
37. Boyle PA, Lei Y, Wilson RS, Leurgans SE, Schneider JA,
Bennett DA. Person-specific contribution of neuropathologies
to cognitive loss in old age. Ann Neurol 2018;83(1):74-83.
38. Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R,
Schneider JA, et al. Attributable risk of Alzheimer's dementia
attributed to age-related neuropathologies. Ann Neurol
2019;85(1):114-24.
39. Jellinger KA, Attems J. Prevalence of dementia disorders
in the oldest-old: an autopsy study. Acta Neuropathol
2010;119:421-33.
40. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan
AM, et al. Toward defining the preclinical stages of Alzheimer’s
disease: Recommendations from the National Institute on
Aging-Alzheimer’s Association workgroups on diagnostic
guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7(3):280-92.
Appendices
References
119
Appendices
41. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox
N, et al. The diagnosis of mild cognitive impairment due to
Alzheimer’s disease: Recommendations from the National
Institute on Aging-Alzheimer’s Association workgroups on
diagnostic guidelines for Alzheimer’s disease. Alzheimers
Dement 2011;7(3):270-9.
42. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR,
Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s
disease: Recommendations from the National Institute on
Aging-Alzheimer’s Association workgroups on diagnostic
guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7(3):263-9.
43. Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA,
Carrillo MC, et al. Introduction to the recommendations from
the National Institute on Aging-Alzheimer’s Association
workgroups on diagnostic guidelines for Alzheimer’s disease.
Alzheimers Dement 2011;7(3):257-62.
44. Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van
der Flier WM, et al. Duration of preclinical, prodromal, and
dementia stages of Alzheimer’s disease in relation to age, sex,
and APOE genotype. Alzheimers Dement 2019;15:888-98.
45. Sperling RA, Donohue MC, Raman R, Sun C-K, Yaari R,
Holdridge K, et al. Association of factors with elevated amyloid
burden in clinically normal older individuals. JAMA Neurol
2020;77(6):735-45.
46. Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E,
Bjerke M et al. CSF and blood biomarkers for the diagnosis of
Alzheimer's disease: A systematic review and meta-analysis.
Lancet Neurol 2016;15(7):673-84.
47. Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U,
Schroder J. Reduced cerebral glucose metabolism in patients
at risk for Alzheimer's disease. Psych Res: Neuroimaging
2007;155:147-54.
48. Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal
NT, Shah RC, et al. Neuropathology of older persons without
cognitive impairment from two community-based studies.
Neurology 2006;66:1837-44.
49. Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve
BF, Ivnik RJ, et al. Neuropathology of cognitively normal
elderly. J Neuropathol Exp Neurol 2003;62:1087-95.
50. Grontvedt GR, Schroder TN, Sando SB, White L, Brathen G,
Doeller CF. Alzheimer’s disease. Curr Bio 2018;28:PR645-9.
51. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli
M, Gloss D, et al. Practice guideline update summary: Mild
cognitive impairment. Neurology 2018;90(3):126-35.
52. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from
prodromal Alzheimer’s disease to Alzheimer’s dementia: A
systematic review of the literature. Dement Geriatr Cogn
Disord Extra 2013;3(1):320-32.
53. Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M,
Mariani C, et al. Spontaneous reversion of mild cognitive
impairment to normal cognition: A systematic review of
literature and meta-analysis J Am Med Dir Assoc
2016;17(10):943-8.
54. Social Security Administration. Minimizing the risk of scams for
people living with dementia. Available at: https://blog.ssa.gov/
minimizing-the-risk-of-scams-for-people-living-with-
dementia/#:~:text=People%20living%20with%20dementia%20
are,struggle%20to%20make%20financial%20decisions.
Accessed October 11, 2023.
55. Memory and Aging Center, UCSF Weill Institute for
Neurosciences. Executive functions. Available at: https://
memory.ucsf.edu/symptoms/executive-functions. Accessed
October 11, 2023.
56. Heerema E. How executive functioning Is affected by dementia.
Verywell Health. Available at: https://www.verywellhealth.com/
executive-functioning-alzheimers-98596. Accessed October
30, 2023.
57. Bioten. Biogen to realign resources for Alzheimer's disease
franchise. Available at: https://investors.biogen.com/news-
releases/news-release-details/biogen-realign-resources-
alzheimers-disease-franchise. Accessed January 31, 2024.
58. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee
M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med
2023;388:9-21.
59. Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P,
Greenberg S, et al. Lecanemab: Appropriate use
recommendations. J Prev Alzheimers Dis. 2023;10(3):362-77.
60. Athar T, Al Balushi K, Khan SA. Recent advances on drug
development and emerging therapeutic agents for Alzheimer's
disease. Mol Biol Rep. 2021;48(7):5629-45.
61. Lao K, Ji N, Zhang X, Qiao W, Tang Z, Gou X. Drug development
for Alzheimer's disease: Review. J Drug Target 2019;27(2):164-73.
62. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F.
Alzheimer’s disease drug development timeline: 2023.
Alzheimers Dement: TRCI 2023. DOI: 10.1002/trc2.12385.
63. Van der Mussele S, Le Bastard N, Saerens J, Somers N, Mariën
P, Goeman J, et al. Agitation-associated behavioral symptoms
in mild cognitive impairment and Alzheimer’s dementia. Aging
Ment Health 2015;19(3):247-57.
64. Ralph SJ, Espinet AJ. Increased all-cause mortality by
antipsychotic drugs: Updated review and meta-analysis in
dementia and general mental health care. J Alzheimers Dis Rep
2018;2:1-26.
65. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J,
Schneider LS, et al. Antipsychotics, other psychotropics, and
the risk of death in patients with dementia: number needed to
harm. JAMA Psychiatry 2015;72:438-45.
66. Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and
Alzheimer's disease: What do we know? Neurodegener Dis
Manag 2014;4(5):351-62.
67. Lloret M-A, Cervera-Ferri A, Nepomuceno M, Monllor P,
Esteve D, Lloret A. Is sleep disruption a cause or consequence
of Alzheimer’s disease? Reviewing its possible role as a
biomarker. Int J Mol Sci 2020;21:1168.
68. Rose KM, Fagin CM, Lorenz R. Sleep Disturbances in Dementia:
What They Are and What To Do. J Gerontol Nurs
2010;36(5):9-14.
69. Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi
M, et al. Comparative efficacy of interventions for aggressive
and agitated behaviors in dementia. Ann Internal Med
2019;171(9):633-42.
70. Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA,
Shah RC, et al. Change in risk of Alzheimer disease over time.
Neurology 2010;75:786-91.
71. National Institute on Aging. Available at: https://www.nia.nih.
gov/health/what-causes-alzheimers-disease. Accessed
December 15, 2023.
72. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop
PH, Pericak-Vance MA, Joo SH, et al. Association of
apolipoprotein E allele epsilon 4 with late-onset familial and
sporadic Alzheimer’s disease. Neurology 1993;43:1467-72.
73. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA,
Mayeux R, et al. Effects of age, sex, and ethnicity on the
association between apolipoprotein E genotype and Alzheimer
disease: A meta-analysis. JAMA 1997;278:1349-56.
74. Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA,
et al. Risk of dementia among white and African American
relatives of patients with Alzheimer disease. JAMA
2002;287(3):329-36.
75. Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for
late-onset Alzheimer’s disease: A population-based, case-
control study. Ann Neurol 1993;33(3):258-66.
76. Mayeux R, Sano M, Chen J, Tatemichi T, Stern Y. Risk of
dementia in first-degree relatives of patients with Alzheimer’s
disease and related disorders. Arch Neurol 1991;48(3):269-73.
77. Lautenschlager NT, Cupples LA, Rao VS, Auerbach SA, Becker
R, Burke J, et al. Risk of dementia among relatives of
Alzheimer’s disease patients in the MIRAGE Study: What is in
store for the oldest old? Neurology 1996;46(3):641-50.
78. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in
the United States (2010-2050) estimated using the 2010
Census. Neurology 2013;80(19):1778-83.
120 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
79. Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA,
et al. Alzheimer's disease is not “brain aging”:
Neuropathological, genetic, and epidemiological human studies.
Acta Neuropathol 2011;121:571-87.
80. Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-
Grau S, Amin N, et al. New insights into the genetic etiology of
Alzheimer’s disease and related dementias. Nat Genet
2022;54:412-36.
81. Loy CT, Schofield PR, Turner AM, Kwok JBJ. Genetics of
dementia. Lancet 2014;383:828-40.
82. Qian J, Wolters FJ, Beiser A, Haan M, Ikram MA, Karlawish J,
et al. APOE-related risk of mild cognitive impairment and
dementia for prevention trials: An analysis of four cohorts.
PLoS Med 2017;14(3):e1002254.
83. Spinney L. Alzheimer’s disease: The forgetting gene. Nature
2014;510(7503):26-8.
84. Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN,
et al. Prevalence of apolipoprotein e4 genotype and
homozygotes (APOE e4/4) among patients diagnosed with
Alzheimer’s disease: A systematic review and meta-analysis.
Neuroepidemiology 2012;38:1-17.
85. Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD,
et al. Utility of the apolipoprotein E genotype in the diagnosis
of Alzheimer’s disease. N Engl J Med 1998;338:506-11.
86. Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC,
Scherr PA, et al. Incidence of Alzheimer disease in a biracial
urban community: Relation to apolipoprotein E allele status.
Arch Neurol 2003;60(2):185-9.
87. Tang M, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al.
The APOE-e4 allele and the risk of Alzheimer disease among
African Americans, whites, and Hispanics. JAMA 1998;279:751-55.
88. Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T,
Aggarwal NT, et al. Cognitive aging in black and white
Americans: Cognition, cognitive decline, and incidence of
Alzheimer disease dementia. Epidemiology 2018;29(1):151-9.
89. Hendrie HC, Murrell J, Baiyewu O, Lane KA, Purnell C,
Ogunniyi A, et al. APOE 4 and the risk for Alzheimer disease
and cognitive decline in African Americans and Yoruba. Int
Psychogeriatr 2014;26(6):977-85.
90. Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS,
et al. Variants in the ATP-binding cassette transporter (ABCA7),
apolipoprotein E epsilon 4, and the risk of late-onset Alzheimer
disease in African Americans. JAMA 2013;309(14):1483-92.
91. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis
SM, et al. Associations between midlife vascular risk factors and
25-year incident dementia in the Atherosclerosis Risk in
Communities (ARIC) cohort. JAMA Neurol 2017;74(10):1246-54.
92. Bakulski KM, Vadari HS, Faul JD, Heeringa SG, Kardia SLR,
Langa KM, et al. Cumulative genetic risk and APOE «4 are
independently associated with dementia status in a multiethnic,
population-based cohort. Neurol Genet 2021;7:e576.
93. Rajan KB, Barnes LL, Wilson RS, McAninch EA, Weuve J,
Sighoko D, et al. Racial differences in the association between
apolipoprotein E risk alleles and overall and total cardiovascular
mortality over 18 years. JAGS 2017;65:2425-30.
94. Kataoka S, Robbins DC, Cowan LD, Go O, Yeh JL, Devereux RB,
et al. Apolipoprotein E polymorphism in American Indians and
its relation to plasma lipoproteins and diabetes. The Strong
Heart Study. Arterioscler Thromb Vasc Biol 1996;16:918-25.
95. Le Guen Y, Raulin AC, Logue MW, Sherva R, Belloy ME, Eger
SJ, et al. Association of African ancestry-specific APOE
missense variant R145C with risk of Alzheimer disease. JAMA
2023;329(7):551-60.
96. Granot-Hershkovitz E, Tarraf W, Kurniansyah N, Daviglus M,
Isasi CR, Kaplan R, et al. APOE alleles’ association with cognitive
function differs across Hispanic/Latino groups and genetic
ancestry in the study of Latinos-investigation of
neurocognitive aging (HCHS/SOL). Alzheimer’s Dement
2021;17:466-74.
97. Lott IT, Dierssen M. Cognitive deficits and associated
neurological complications in individuals with Down’s
syndrome. Lancet Neurol 2010;9(6):623-33.
98. National Down Syndrome Society. Alzheimer’s Disease and
Down Syndrome. Available at: https://www.ndss.org/resources/
alzheimers/. Accessed December 15, 2023.
99. Fortea J, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L,
Barroeta I, et al. Clinical and biomarker changes of Alzheimer's
disease in adults with Down syndrome: A cross-sectional study.
Lancet 2020;395(10242):1988-97.
100. Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona-
Iragui M. Alzheimer's disease associated with Down syndrome:
A genetic form of dementia. Lancet Neurol 2021;20(11):930-42.
101. Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher
EMC, et al. Association of dementia with mortality among
adults with Down syndrome older than 35 years. JAMA Neurol
2019;76(2):152-60.
102. Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer
disease. J Geriatr Psychiatry Neurol 2010;23(4):213-27.
103. Goldman JS, Hahn SE, Bird T. Genetic counseling and testing
for Alzheimer disease: Joint practice guidelines of the American
College of Medical Genetics and the National Society of
Genetic Counselors. Genet Med 2011;13:597-605.
104. Lopera F, Marino C, Chandrahas AS, O'Hare M, Villalba-Moreno
ND, Aguillon D, et al. Resilience to autosomal dominant
Alzheimer's disease in a Reelin-COLBOS heterozygous man.
Nat Med 2023 May;29(5):1243-52.
105. Arboleda-Velasquez JF, Lopera F, O'Hare M, Delgado-Tirado S,
Marino C, Chmielewska N, et al. Resistance to autosomal
dominant Alzheimer's disease in an APOE3 Christchurch
homozygote: a case report. Nat Med 2019;25(11):1680-83.
106. Wolters FJ, van der Lee SJ, Koudstaal PJ, van Duijn CM,
Hofman A, Ikam MK, et al. Parental family history of dementia
in relation to subclinical brain disease and dementia risk.
Neurology 2017;88:1642-9.
107. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C,
Banerjee S, et al. Dementia prevention, intervention, and care:
2020 report of the Lancet Commission. Lancet 2020; 396
(10248):413-46.
108. Rosenwohl-Mack A, Yaffe K, Carrasco A, Hoffmann CM, Barnes
DE. Risk factors associated with Alzheimer disease and related
dementias by sex and race and ethnicity in the US. JAMA
Neurol 2022;79(6):584-91.
109. World Health Organization. Risk reduction of cognitive decline
and dementia: WHO guidelines. https://www.who.int/
publications/i/item/risk-reduction-of-cognitive-decline-and-
dementia. Accessed December 15, 2023.
110. Institute of Medicine. Cognitive Aging: Progress in
Understanding and Opportunity for Action. Washington, D.C.:
The National Academies Press; 2015.
111. James BD, Bennett DA. Causes and patterns of dementia: An
update in the era of redefining Alzheimer's disease. Ann Rev
Public Health. 2019;40:65-84.
112. Mergenthaler P, Lindauer U, GA Dienel, Meisel A. Sugar for the
brain: The role of glucose in physiological and pathological
brain function. Trends Neurosci 2013;36(10):587-97.
113. Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC,
Llewellyn DJ. Stroke and dementia risk: A systematic review
and meta-analysis. Alzheimers Dement 2018;14(11):1416-26.
114. Samieri C, Perier MC, Gaye B, Proust-Lima C Helmer C,
Dartigues JF, et al. Association of cardiovascular health level in
older age with cognitive decline and incident dementia. JAMA
2018;320(7):657-64.
115. Rönnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk
factors and dementia: 40-year follow-up of a population-based
cohort. Dement Geriatr Cogn Disord 2011;31(6):460-6.
116. Abell JG, Kivimäki M, Dugravot A, Tabak AG, Fayosse A, Shipley
M, et al. Association between systolic blood pressure and
dementia in the Whitehall II cohort study: Role of age, duration,
and threshold used to define hypertension. Eur Heart J
2018;39(33):3119-25.
117. Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C,
et al. Midlife vascular risk factor exposure accelerates structural
brain aging and cognitive decline. Neurology 2011;77:461-8.
121
Appendices
118. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus
and risk of dementia: A meta-analysis of prospective
observational studies. Diabetes Investig 2013;4(6):640-50.
119. Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for
Alzheimer’s disease: The confounders, interactions, and
neuropathology associated with this relationship. Epidemiol
Rev 2013;35(1):152-60.
120. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer
disease. Nat Rev Neurol 2011;7(3):137-52.
121. Johnson AL, Nystrom NC, Piper ME, Cook J, Norton DL,
Zuelsdorff M, et al. Cigarette smoking status, cigarette
exposure, and duration of abstinence predicting incident
dementia and death: A multistate model approach. J Alzheimers
Dis 2021;80(3):1013-23.
122. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C.
Smoking, dementia and cognitive decline in the elderly, a
systematic review. BMC Geriatr 2008;8:36.
123. Ogino E, Manly JJ, Schupf N, Mayeux R, Gu Y. Current and past
leisure time physical activity in relation to risk of Alzheimer’s
disease in older adults. Alzheimers Dement 2019;15(12):1603-11.
124. Najar J, Ostling S, Gudmundsson P, Sundh V, Johansson L, Kern
S, et al. Cognitive and physical activity and dementia: A 44-year
longitudinal population study of women. Neurology
2019;92(12):e1322-e1330.
125. Buchman AS, Yu L, Wilson RS, Lim A, Dawe RJ, Gaiteri C, et al.
Physical activity, common brain pathologies, and cognition in
community-dwelling older adults. Neurology
2019;92(8):e811-e822.
126. Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan
RS, et al. Physical activity, brain volume, and dementia risk: The
Framingham Study. J Gerontol A Biol Sci Med Sci 2017;72:789-95.
127. Stephen R, Hongistro K, Solomon A, Lonnroos E. Physical
activity and Alzheimer’s disease: A systematic review. J
Gerontol A Biol Sci Med Sci 2017;72(6):733-9.
128. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical
activity prevent cognitive decline and dementia? A systematic
review and meta-analysis of longitudinal studies. BMC Public
Health 2014;14:510.
129. Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of physical
activity on cognitive decline, dementia, and its subtypes:
Meta-analysis of prospective studies. Biomed Res Int
2017;2017:9016924.
130. Jensen CS, Simonsen AH, Siersma V, Beyer N, Frederiksen KS,
Gottrup H, et al. Patients with Alzheimer’s disease who carry
the APOE e4 allele benefit more from physical exercise. TRCI
2019;5:99-106.
131. Felisatti F, Gonneaud J, Palix C, Garnier-Crussard A, Mézenge
F, Brigitte L, et al. Role of cardiovascular risk factors on the
association between physical activity and brain integrity
markers in older adults. Neurology 2022;98(20):e2023-e2035.
132. Casaletto K, Ramos-Miguel A, VandeBunte A, Memel M,
Buchman A, Bennett D et al. Late-life physical activity relates
to brain tissue synaptic integrity markers in older adults.
Alzheimers Dement 2022;18(11):2023-35.
133. Nguyen S, LaCroix AZ, Hayden KM, Di C, Palta P, Stefanick ML,
et al. Accelerometermeasured physical activity and sitting with
incident mild cognitive impairment or probable dementia
among older women. Alzheimers Dement 2023; DOI: 10.1002/
alz.12908.
134. Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas
A. Adherence to a Mediterranean-style diet and effects on
cognition in adults: A qualitative evaluation and systematic
review of longitudinal and prospective trials. Front Nutr
2016;3:22.
135. Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA,
Ukoumunne OC, et al. Mediterranean diet, cognitive function,
and dementia: A systematic review. Epidemiology 2013;24:479-89.
136. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett
DA, et al. MIND diet slows cognitive decline with aging.
Alzheimers Dement 2015;11(9):1015-22.
137. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA,
Aggarwal NT. MIND diet associated with reduced incidence of
Alzheimer’s disease. Alzheimers Dement 2015;11:1007-14.
138. Van den Brink AC, Brouwer-Broisma EM, Berendsen AAM van
de Rest O. The Mediterranean, Dietary Approaches to Stop
Hypertension (DASH), and Mediterranean-DASH Intervention
for Neurodegenerative Delay (MIND) Diets are associated with
less cognitive decline and a lower risk of Alzheimer's disease: A
review. Adv Nutr 2019;10:1040-65.
139. Ballarini T, Melo van Lent D, Brunner J, Schroder A,
Wolfsgruber S, Altenstein S, et al. Mediterranean diet,
Alzheimer disease biomarkers and brain atrophy in old age.
Neurology 2021;96(24):e2920-e2932.
140. Agarwal P, Leurgans SE, Agrawal S, Aggarwal NT, Cherian LJ,
James BD, et al. Association of Mediterranean-DASH
Intervention for Neurodegenerative Delay and Mediterranean
Diets With Alzheimer Disease Pathology. Neurology
2023;100(22):e2259-68.
141. Barnes LL, Dhana K, Liu X, Carey VJ, Ventrelle J, Johnson K,
et al. Trial of the MIND Diet for prevention of cognitive decline
in older persons. N Engl J Med 2023;389(7):602-11.
142. Grant WB, Steven M. Diet’s role in modifying risk of
Alzheimer’s disease: History and present understanding.
J Alzheimer's Dis 2023;96(4):1353-82.
143. Martinez-Gonzalez MA, Gea A, Ruiz-Canela M. The
Mediterranean diet and cardiovascular health: A critical review:
Circulation Res 2019;124:779-98.
144. Sanches Machado d’Almeida K, Spillere SR, Zuchinali P, Souza
GC. Mediterranean diet and other dietary patterns in primary
prevention of heart failure and changes in cardiac function
markers: A systematic review. Nutrients 2018;10:58. Doi:
10.3390/nu10010058.
145. Walker ME, O’Donnell AA, Himali JJ, Rajendran I, van Lent DM,
Ataklte F, et al. Associations of the Mediterranean-Dietary
Approaches to Stop Hypertension Intervention for
Neurodegenerative Delay diet with cardiac remodelling in the
community: The Framingham Heart Study. Br J Nutr
2021;126(12):1888-96.
146. Butler M, Nelson VA, Davila H, Ratner E, Fink HA Hemmy LS,
et al. Over-the-counter supplement interventions to prevent
cognitive decline, mild cognitive impairment, and clinical
Alzheimer-type dementia. Ann Intern Med 2018;168:52-62.
147. Kivimaki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, Nyberg
ST, et al. Body mass index and risk of dementia: Analysis of
individual-level data from 1.3 million individuals. Alzheimers
Dement 2018;14:601-9.
148. Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta
N, et al. Association between midlife vascular risk factors and
estimated brain amyloid deposition. JAMA 2017;17(14):1443-50.
149. Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence
on the association between serum cholesterol and risk of
late-life dementia: Review and meta-analysis. J Alzheimers Dis
2017;56(1):215-28.
150. Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM.
Contribution of Socioeconomic Status at 3 Life-Course
Periods to Late-Life Memory Function and Decline: Early and
Late Predictors of Dementia Risk. Am J Epidemiol
2017;186(7):805-14.
151. Caunca MR, Odden MC, Glymour MM, Elfassy T, Kershaw KN,
Sidney S, Yaffe K, Launer L, Zeki Al Hazzouri A. Association of
Racial Residential Segregation Throughout Young Adulthood
and Cognitive Performance in Middle-aged Participants in the
CARDIA Study. JAMA Neurol 2020;77(8):1000-7.
152. Chen R, Williams DR, Nishimi K, Slopen N, Kubzansky LD,
Weuve J. A life course approach to understanding stress
exposures and cognitive function among middle-aged and
older adults. Soc Sci Med 2022;314:115448.
153. George KM, Lutsey PL, Kucharska-Newton A, Palta P, Heiss G,
Osypuk T, Folsom AR. Life-Course Individual and
Neighborhood Socioeconomic Status and Risk of Dementia in
the Atherosclerosis Risk in Communities Neurocognitive Study.
Am J Epidemiol 2020;189(10):1134-42.
122 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
154. George KM, Gilsanz P, Peterson RL, Barnes LL, DeCarli CS,
Mayeda ER, Mungas DM, Whitmer RA. Impact of
Cardiovascular Risk Factors in Adolescence, Young Adulthood,
and Midlife on Late-Life Cognition: Study of Healthy Aging in
African Americans. J Gerontol A Biol Sci Med Sci
2021;76(9):1692-8.
155. Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W,
Breitner JC, et al. Incidence and prevalence of dementia in the
Cardiovascular Health Study. J Am Geriatr Soc
2004;52(2):195-204.
156. Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L,
Schellenberg GD, et al. Dementia and Alzheimer disease
incidence: A prospective cohort study. Arch Neurol
2002;59(11):1737-46.
157. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease.
Lancet Neurol 2012;11(11):1006-12.
158. Sando SB, Melquist S, Cannon A, Hutton M, Sletvold O,
Saltvedt I, et al. Risk-reducing effect of education in Alzheimer’s
disease. Int J Geriatr Psychiatry 2008;23(11):1156-62.
159. Hendrie HC, Smith-Gamble V, Lane KA, Purnell C, Clark DO,
Gao S. The Association of early life factors and declining
incidence rates of dementia in an elderly population of
African Americans. J Gerontol B Psychol Sci Soc Sci
2018;16(73, suppl 1):S82-9.
160. Manly JJ, Jones RN, Langa KM, Ryan LH, Levine DA,
McCammon R, et al. Estimating the Prevalence of Dementia
and Mild Cognitive Impairment in the US: The 2016 Health and
Retirement Study Harmonized Cognitive Assessment Protocol
Project. JAMA Neurol 2022;79(12):1242-9.
161. Rawlings AM, Sharrett AR, Mosley TH, Wong DF, Knopman DS,
Gottesman RF. Cognitive reserve in midlife is not associated
with amyloid- deposition in late-life. J Alzheimers Dis
2019:517-21.
162. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett
DA. Education and cognitive reserve in old age. Neurology
2019;92(10):e1041-50.
163. Stern Y. What is cognitive reserve? Theory and research
application of the reserve concept. J Int Neuropsychol Soc
2002;8:448-60.
164. Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S,
Cantilon M, Chetelat G, et al. Whitepaper: Defining and
investigating cognitive reserve, brain reserve, and brain
maintenance. Alzheimers Dement 2020;16(9):1305-11.
165. Grzywacz JG, Segel-Karpas D, Lachman ME. Workplace
exposures and cognitive function during adulthood: Evidence
from National Survey of Midlife Development and the O*NET.
J Occup Environ Med 2016;58(6):535-41.
166. Pool LR, Weuve J, Wilson RS, Bültmann U, Evans DA, Mendes
de Leon CF. Occupational cognitive requirements and late-life
cognitive aging. Neurology 2016;86(15):1386-92.
167. Then FS, Luck T, Luppa M, Arelin K, Schroeter ML, Engel C,
et al. Association between mental demands at work and
cognitive functioning in the general population: Results of the
health study of the Leipzig Research Center for Civilization
Diseases. J Occup Med Toxicol 2014;9:23.
168. Fisher GG, Stachowski A, Infurna FJ, Faul JD, Grosch J, Tetrick
LE. Mental work demands, retirement, and longitudinal
trajectories of cognitive functioning. J Occup Health Psychol
2014;19(2):231-42.
169. Coleman ME, Roessler MEH, Peng S, Roth AR, Risacher SL,
Saykin AJ, et al. Social enrichment on the job: Complex work
with people improves episodic memory, promotes brain
reserve, and reduces the risk of dementia. Alzheimers Dement
2023;19(6):2655-65.
170. Soh Y, Eng CW, Mayeda ER, Whitmer RA, Lee C, Peterson RL,
et al. Association of primary lifetime occupational cognitive
complexity and cognitive decline in a diverse cohort: Results
from the KHANDLE study. Alzheimers Dement
2023;19(9):3926-35.
171. McDowell I, Xi G, Lindsay J, Tierney M. Mapping the
connections between education and dementia. J Clin Exp
Neuropsychol 2007;29(2):127-41.
172. Harris CD, Watson KB, Carlson SA, Fulton JE, Dorn JM,
Elam-Evans L. Adult participation in aerobic and muscle-
strengthening physical activities — United States, 2011. Morb
Mortal Wkly Rep 2013;62(17):326-30.
173. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and
trends in diabetes among adults in the United States,
1988-2012. JAMA 2015;314(10):1021-9.
174. Sims M, Diez Roux AV, Boykin S, Sarpong D, Gebreab SY, Wyatt
SB, et al. The socioeconomic gradient of diabetes prevalence,
awareness, treatment, and control among African Americans in
the Jackson Heart Study. Ann Epidemiol 2011;21(12):892-8.
175. Lee TC, Glynn RJ, Peña JM, Paynter NP, Conen D, Ridker PM,
et al. Socioeconomic status and incident type 2 diabetes
mellitus: Data from the Women's Health Study. PLoS One
2011;6(12):E27670.
176. Gillespie CD, Hurvitz KA. Prevalence of hypertension and
controlled hypertension — United States, 2007–2010.
MMWR Suppl 2013;62(3):144-8.
177. Centers for Disease Control and Prevention. Current
Cigarette Smoking Among Adults in the United States.
Available at: https://www.cdc.gov/tobacco/data_statistics/
fact_sheets/adult_data/cig_smoking/index.htm. Accessed
December 15, 2023.
178. Kweon H, Aydogan G, Dagher A, Bzdok D, Ruff CC, Nave G,
et al. Human brain anatomy reflects separable genetic and
environmental components of socioeconomic status. Sci Adv
2022;8(20):eabm2923.
179. Weuve J, Bennett EE, Ranker L, Gianattasio KZ, Pedde M, Adar
SD, et al. Exposure to air pollution in relation to risk of
dementia and related outcomes: An updated systematic review
of the epidemiologic literature. Environ Health Perspect
2021;129(9):96001.
180. Bernard SM, McGeehin MA. Prevalence of blood lead levels
>or= 5 micro g/dL among US children 1 to 5 years of age and
socioeconomic and demographic factors associated with blood
of lead levels 5 to 10 micro g/dL, Third National Health and
Nutrition Examination Survey, 1988-1994. Pediatrics
2003;112(6 Pt 1):1308-13.
181. Griffith M, Tajik M, Wing S. Patterns of agricultural pesticide
use in relation to socioeconomic characteristics of the
population in the rural U.S. South. Int J Health Serv
2007;37(2):259-77.
182. Staff RT, Hogan MJ, Williams DS, Whalley LJ. Intellectual
engagement and cognitive ability in later life (the “use it or lose
it” conjecture): Longitudinal, prospective study. BMJ
2018;363:k4925.
183. Karp A, Paillard-Borg S, Wang H-X, Silverstein M, Winblad B,
Fratiglioni L. Mental, physical and social components in leisure
activities equally contribute to decrease dementia risk. Dement
Geriatr Cogn Disord 2005;21(2):65-73.
184. Di Marco LY, Marzo A, Muñoz-Ruiz M, Ikram MA, Kivipelto M,
Ruefenacht D, et al. Modifiable lifestyle factors in dementia: A
systematic review of longitudinal observational cohort studies.
J Alzheimers Dis 2014;42(1)119-35.
185. James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social
activity and cognitive decline in old age. J Int Neuropsychol Soc
2011;17(6):998-1005.
186. Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure
activities and future risk of cognitive impairment and dementia:
Systematic review and meta-analysis. Int Psychogeriatr
2016;9:1-16.
187. Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J.
Cognitive activities delay onset of memory decline in persons
who develop dementia. Neurology 2009;73:356-61.
188. Sanjeev G, Weuve J, Jackson JW, VanderWeele TJ, Bennett
DA, Grodstein F, et al. Late-life cognitive activity and dementia.
Epidemiology 2016;27(5):732-42.
189. Wang Z, Marseglia A, Shang Y, Dintica C, Patrone C, Xu W.
Leisure activity and social integration mitigate the risk of
dementia related to cardiometabolic diseases: A population-
based longitudinal study. Alzheimer’s Dement. 2020;16:316-25.
123
Appendices
190. Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, Livingston G.
Association of social contact with dementia and cognition:
28-year follow-up of the Whitehall II cohort study. PLoS Med
2019;16(8):e1002862.
191. Centers for Disease Control and Prevention. Surveillance
Report: Traumatic Brain Injury-Related Deaths by Age Group,
Sex, and Mechanism of Injury. Available at: https://www.cdc.
gov/traumaticbraininjury/pdf/TBI-surveillance-
report-2018-2019-508.pdf. Accessed December 15, 2023.
192. Fann JR, Ribe AR, Pedersen HS, Fenger-Gron M, Christensen
J, Benros ME, et al. Long-term risk of dementia among people
with traumatic brain injury in Denmark: A population-based
observational cohort study. Lancet Psychiatry 2018;5(5):424-31.
193. LoBue C, Munro C, Schaffert J, Didehbani N, Hart J, Batjer H,
et al. Traumatic brain injury and risk of long-term brain
changes, accumulation of pathological markers, and developing
dementia: A review. J Alzheimers Dis 2019;70(3):629-54.
194. Schneider ALC, Selvin E, Latour L, Turtzo LC, Coresh J, Mosley
T, et al. Head injury and 25-year risk of dementia. Alzheimer’s
Dement 2021;17:1432-41.
195. Centers for Disease Control and Prevention. TBI Data. Available
at: https://www.cdc.gov/traumaticbraininjury/data/index.html.
Accessed December 15, 2023.
196. Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN,
Drosdick D, et al. Documented head injury in early adulthood
and risk of Alzheimer’s disease and other dementias. Neurology
2000;55(8):1158-66.
197. Teasdale G, Jennett B. Assessment of coma and impaired
consciousness: A practical scale. Lancet 1974;2(7872):81-4.
198. Centers for Disease Control and Prevention. Traumatic Brain
Injury & Concussion. Potential Effects. Available at: https://
www.cdc.gov/traumaticbraininjury/outcomes.html. Accessed
December 15, 2023.
199. Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia
resulting from traumatic brain injury: what is the pathology?
Arch Neurol 2012;69(10):1245-51.
200. LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J Jr,
Womack KB. Traumatic brain injury history is associated with
earlier age of onset of Alzheimer disease. Clin Neuropsychol
2017;31(1):85-98.
201. Schaffert J, LoBue C, White CL, Chiang H-S, Didehboni N,
Lacritz L, et al. Traumatic brain injury history is associated with
an earlier age of dementia onset in autopsy-confirmed
Alzheimer's disease. Neuropsychology 2018 May;32(4):410-16.
202. Stein TD, Montenigro PH, Alvarez VE, Xia W, Crary JF, Tripodis
Y, et al. Beta-amyloid deposition in chronic traumatic
encephalopathy. Acta Neuropathol 2015;130(1):21-34.
203. Turk KW, Geada A, Alvarez VE, Xia W, Cherry JD, Nicks R, et al.
A comparison between tau and amyloid-beta cerebrospinal fluid
biomarkers in chronic traumatic encephalopathy and Alzheimer
disease. Alz Res Therapy 2022;14:28. Available at: https://doi.
org/10.1186/s13195-022-00976-y. Accessed December 13, 2023.
204. Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, Bauer RM.
Research gaps and controversies in chronic traumatic
encephalopathy: A review. JAMA Neurol 2017;74(10):1255-62.
205. Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco
ML, Kiernan PT, et al. Duration of American football play and
chronic traumatic encephalopathy. Ann Neurol
2020;87(1):116-31.
206. Nowinski CJ, Bureau SC, Buckland ME, Curtis MA, Daneshvar
DH, Faull RLM, et al. Applying the Bradford Hill Criteria for
Causation to Repetitive Head Impacts and Chronic Traumatic
Encephalopathy. Front Neurol 2022;13:938163.
207. Shi L, Chen S, Ma M, Bao Y, Han Y, Wang Y, et al. Sleep
disturbances increase the risk of dementia: A systematic review
and meta-analysis. Sleep Med Rev 2018;40:4-16.
208. Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C,
Sommerlad A. Association of sleep duration in middle and old
age with incidence of dementia. Nat Commun 2021;12(1):2289.
209. Winer JR, Keters KD, Kennedy G, Jin M, Goldstein-Piekarski A,
Poston KL, et al. Association of short and long sleep duration
with amyloid-β burden and cognition in aging. JAMA Neurol
2021;78(10):1187-96.
210. Makin S. Deep sleep gives your brain a deep clean: Slow-wave
activity during dreamless slumber helps wash out neural
detritus Scientific American Nov 1, 2019. Available at: https://
www.scientificamerican.com/article/deep-sleep-gives-your-
brain-a-deep-clean1/. Accessed December 15, 2023.
211. Insel PS, Mohlenhoff BS, Neylan TC, Krystal AD, Mackin RS.
Association of sleep and -amyloid pathology among older
cognitively unimpaired adults. JAMA Netw Open
2021;4(7):e2117573.
212. Andrade A, Bubu OM, Varga AW, Osorio RS. The relationship
between obstructive sleep apnea and Alzheimer’s disease. J
Alzheimers Dis 2018; 64(Suppl 1):S255-S270.
213. Bubu, OM, Andrade AA, Umasabor-Bubu U, Hogan MH, Turner
AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and
Alzheimer's disease: A systematic review integrating three
decades of multidisciplinary research. Sleep Med Rev
2020;50:101250.
214. Wang C, Holtzman DM. Bidirectional relationship between
sleep and Alzheimer’s disease: Role of amyloid, tau, and other
factors. Neuropsychopharmacol 2020;45:104–20.
215. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease
pathology--a bidirectional relationship. Nat Rev Neurol
2014;10(2):115-9.
216. Lucey BP. It's complicated: The relationship between sleep
and Alzheimer's disease in humans. Neurobiol Dis
2020:144:105031.
217. Ju Y-ES, McLeland JS, Toedebusch CD, Xiong C, Fagan AM,
Duntley SP, Morris JC, et al. Sleep quality and preclinical
Alzheimer disease. JAMA Neurol 2013;70(5):587–93.
218. Lao XQ, Liu X, Deng HB, Chan TC, Ho KF, Wang F, et al. Sleep
quality, Sleep duration, and the risk of coronary heart disease:
A prospective cohort study with 60,586 adults. J Clin Sleep
Med 2018;14(1):109-17.
219. U.S. Environmental Protection Agency. 2019. Integrated
science assessment (ISA) for particulate matter (final report,
2019). EPA/600/R-19/188. Washington, DC.
220. Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air
Pollution and Dementia: A Systematic Review. J Alzheimers
Dis 2019;70(s1):S145-S163.
221. Zhang B, Weuve J, Langa KM, D’Souza J, Szpiro A, Faul J, et al.
Comparison of particulate air pollution from different emission
sources and incident dementia in the U.S. JAMA Intern Med
2023;10.1001/jamainternmed.2023.3300.
222. Abolhasani E, Hachinski V, Ghazaleh N, Azarpazhooh MR,
Mokhber N, Martin J. Air pollution and Incidence of dementia:
A systematic review and meta-analysis. Neurology 2022;DOI:
10.1212/WNL.0000000000201419.
223. Sprung J, Knopman DS, Petersen RC, Mielke MM, Weingarten
TN, Vassilaki M, et al. Association of hospitalization with
long-term cognitive trajectories in older adults. J Am Geriatr
Soc 2021;69(3):660-8.
224. James BD, Wilson RS, Capuano AW, Boyle PA, Shah RC, Lamar
M, et al. Cognitive decline after elective and nonelective
hospitalizations in older adults. Neurology
2019;92(7):e690-e699.
225. Brown CH, Sharrett AR, Coresh J, Schneider ALC, Alonso A,
Knopman DS, et al. Association of hospitalization with
long-term cognitive and brain MRI changes in the ARIC
cohort. Neurology 2015;84:1443-53.
226. Pandharipande PP, Girard TD, Jackson JC, Morandi A,
Thompson JL, Pun BT, et al. Long-term cognitive impairment
after critical illness. N Engl J Med 2013;369(14):1306-16.
227. Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJPA, Carson
SS, Curtis JR, et al. Association between acute care and critical
illness hospitalization and cognitive function in older adults.
JAMA 2010;303(8):763-70.
228. Mart MF, Pun BT, Pandharipande P, Jackson JC, Ely EW. ICU
survivorship - The Relationship of delirium, sedation,
dementia, and acquired weakness. Crit Care Med
2021;49(8):1227-40.
229. James BD, Wilson RS, Capuano AW, Boyle PA, Shah RC, Lamar
M, et al. Hospitalization, Alzheimer's Disease and Related
Neuropathologies, and Cognitive Decline. Ann Neurol
2019;86(6):844-52.
124 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
230. Fong TG, Inouye SK. The inter-relationship between delirium
and dementia: The importance of delirium prevention. Nat Rev
Neurol 2022;18(10):579-96.
231. Kim JE, Tamres LK, Orbell SL, Butters MA, McDade E, Lingler
JH, et al. “And does that necessarily mean absolutely
Alzheimer’s?” An Analysis of questions raised following amyloid
PET results disclosure. Am J Geriat Psychiat. Published August
13, 2023. DOI:https://doi.org/10.1016/j.jagp.2023.08.005.
232. Largent EA, Bradbury AR. Bringing Alzheimer disease testing
and results disclosure Into the 21st Century Cures Act. JAMA
Neurol 2022;79(3):219-20.
233. Erickson CM, Clark LR, Ketchum FB, Chin NA, Gleason CE
Largent EA. Implications of preclinical Alzheimer’s disease
biomarker disclosure for US policy and society. Alzheimers
Dement: DADM 2022. DOI 10.1002/dad2.12339.
234. Barnes LL. Alzheimer disease in African American individuals:
increased incidence or not enough data? Nature reviews.
Neurology. Jan 2022;18(1):56-62.
235. Administration for Community Living. 2020 Profile of Older
Americans: May 2021. Available at: https://acl.gov/sites/default/
files/Aging%20and%20Disability%20in%20
America/2020ProfileOlderAmericans.Final_.pdf. Accessed
December 15, 2023.
236. Gilmore-Bykovskyi A, Croff R, Glover CM, Jackson JD,
Resendez J, Perez A, et al. Traversing the aging research and
health equity divide: Toward intersectional frameworks of
research justice and participation. Gerontologist
2022;62(5):711-20.
237. U.S. Census Bureau. 2023 National Population Projections:
Downloadable Files. Available at: https://www.census.gov/data/
tables/2023/demo/popproj/2023-summary-tables.html.
Accessed December 15, 2023.
238. Administration on Aging, Administration for Community Living,
U.S. Department of Health and Human Services. A Profile of
Older Americans: 2016. Available at: https://www.acl.gov/sites/
default/files/Aging%20and%20Disability%20in%20
America/2016-Profile.pdf. Accessed December 15, 2023.
239. Guerreiro R, Bras J. The age factor in Alzheimer's disease.
Genome Med 2015;7:106.
240. Hudomiet P, Hurd MD, Rohwedder S. Trends in inequalities in
the prevalence of dementia in the United States. Proc Natl
Acad Sci USA 2022;119(46):e2212205119.
241. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans
DA. Population estimate of people with clinical AD and mild
cognitive impairment in the United States (2020-2060).
Alzheimers Dement 2021;17(12):1966-75.
242. Hendriks S, Peetoom K, Bakker C, van der Flier WM, Papma
JM, Koopmans R, et al. Global prevalence of young-onset
dementia: A systematic review and meta-analysis. JAMA Neurol
2021;78(9):1080-90.
243. James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA,
Schneider JA. TDP-43 stage, mixed pathologies, and clinical
Alzheimer’s-type dementia. Brain 2016;139(11):2983-93.
244. Serrano-Pozo A, Qian J, Monsell SE, Blacker D, Gomez-Isla T,
Betensky RA, et al. Mild to moderate Alzheimer dementia with
insufficient neuropathological changes. Ann Neurol
2014;75:597-601.
245. Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z,
James BD, et al. Mixed pathology is more likely in black than
white decedents with Alzheimer dementia. Neurology
2015;85:528-34.
246. Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman
DS, et al. Mild cognitive impairment due to Alzheimer disease in
the community. Ann Neurol. Aug 2013;74(2):199-208.
247. Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I,
Hanna L, et al. Association of amyloid positron emission
tomography with subsequent change in clinical management
among Medicare beneficiaries with mild cognitive impairment
or dementia. JAMA 2019;321(13):1286-94.
248. U.S. Census Bureau. 2023 National Populaiton Prpojections
Tables: Main Series. Available at: https://www.census.gov/data/
tables/2023/demo/popproj/2023-summary-tables.html.
Accessed December 15, 2023.
249. Jack CR Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman
DS, Vemuri P, et al. Prevalence of biologically vs clinically
defined Alzheimer spectrum entities using the National
Institute on Aging-Alzheimer's Association Research
Framework. JAMA Neurol 2019;76(10):1174-83.
250. Gustavsson A, Norton N, Fast T, Frölich L, Georges J, Holzapfel
D. Global estimates on the number of persons across the
Alzheimer's disease continuum. Alzheimers Dement 2022;doi:
10.1002/alz.12694.
251. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting
the prevalence of preclinical and clinical Alzheimer's disease in
the United States. Alzheimers Dement 2018;14(2):121-9.
252. Kotagal V, Langa KM, Plassman BL, Fisher GG, Giordani BJ,
Wallace RB, et al. Factors associated with cognitive evaluations
in the United States. Neurology 2015;84(1):64-71.
253. Taylor DH, Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The
accuracy of Medicare claims as an epidemiological tool: The
case of dementia revisited. J Alzheimers Dis 2009;17(4):807-15.
254. Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power
MC. Racial disparities and temporal trends in dementia
misdiagnosis risk in the United States. Alzheimer's & dementia.
2019;5:891-8.
255. Lang L, Clifford A, Wei L, Zhang D, Leung D, Augustine G, et al.
Prevalence and determinants of undetected dementia in the
community: A systematic literature review and a meta-analysis.
BMJ Open 2017;7(2):e011146.
256. Lin PJ, Daly AT, Olchanski N, Cohen JT, Neumann PJ, Faul JD,
Fillit HM, Freund KM. Dementia diagnosis disparities by race
and ethnicity. Med Care 2021;59(8):679-86.
257. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus
QM. Underdiagnosis of dementia: An observational study of
patterns in diagnosis and awareness in US older adults. J Gen
Intern Med 2018;33(7):1131-8.
258. Nguyen HQ, Borson S, Khang P, Langer-Gould A, Wang SE,
Carrol J, et al. Dementia diagnosis and utilization patterns in a
racially diverse population within an integrated health care
delivery system. Alzheimers Dement (N Y). 2022 Mar
13;8(1):e12279.
259. Grodstein F, Chang C-H, Capuano AW, Power MC, Marquez
DX, Barnes LL. Identification of Dementia in Recent Medicare
Claims Data, Compared With Rigorous Clinical Assessments.
J Gerontol A Biol Sci Med Sci 2022;77(6):1272-8.
260. Grodstein F, James BD, Chen Y, Capuano AW, Power MC,
Bennett DA, et al. Identification of dementia in Medicare claims
compared to rigorous clinical assessments in African
Americans. J Gerontol A Biol Sci Med Sci. 2023; Sep
30:glad235. https://doi.org/10.1093/gerona/glad235.
261. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of
dementia in the US population using Medicare claims: Insights
from linked survey and administrative claims data. Alzheimers
Dement (N Y). 2019;5:197-207.
262. Healthy People 2030. Available at: https://health.gov/
healthypeople/objectives-and-data/browse-objectives/
dementias/increase-proportion-older-adults-dementia-or-
their-caregivers-who-know-they-have-it-dia-01. Accessed
December 15, 2023.
263. Barrett AM, Orange W, Keller M, Damgaard P, Swerdlow RH.
Short-term effect of dementia disclosure: How patients and
families describe the diagnosis. J Am Geriatr Soc
2006;54(12):1968-70.
264. Zaleta AK, Carpenter BD, Porensky EK, Xiong C, Morris JC.
Agreement on diagnosis among patients, companions, and
professionals after a dementia evaluation. Alzheimer Dis Assoc
Disord 2012;26(3):232-7.
265. Amjad H, Roth DL, Samus QM, Yasar S, Wolff JL. Potentially
unsafe activities and living conditions of older adults with
dementia. J Am Geriatr Soc 2016;64(6):1223-32.
266. Alzheimer’s Association. 2015 Alzheimer’s Disease Facts and
Figures. Special report: Disclosing a diagnosis of Alzheimer's
disease. Available at: https://www.alzheimersanddementia.com/
article/S1552-5260(15)00058-8/fulltext. Accessed December
15, 2023.
125
Appendices
267. Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and
Figures. Special report: Alzheimer's detection in the primary
care setting — connecting patients with physicians. Available
at: https://www.alzheimersanddementia.com/article/S1552-
5260(19)30031-7/fulltext. Accessed December 15, 2023.
268. Qian Y, Chen X, Tang D, Kelley AS, Li J. Prevalence of
memory-related diagnoses among U.S. older adults with early
symptoms of cognitive impairment. J Gerontol A;76(10):1846-53.
269. Power MC, Willens V, Prather C, Moghtaderi A, Chen Y,
Gianattasio KZ, et al. Risks and benefits of clinical diagnosis
around the time of dementia onset. Gerontol Geriatr Med
2023;9:23337214231213185.
270. Spargo D, Zur R, Lin PJ, Synnott P, Klein E, Hartry A. Estimating
prevalence of early symptomatic Alzheimer's disease in the
United States. Alzheimers Dement (Amst) 2023;15(4):e12497.
271. Liu Y, Jun H, Becker A, Wallick C, Mattke S. Detection rates of
mild cognitive impairment in primary care for the United States
Medicare population. J Prev Alzheimers Dis 2023. Available at:
https://link.springer.com/article/10.14283/jpad.2023.131.
Accessed December 15, 2023.
272. Reisberg B, Gauthier S. Current evidence for subjective
cognitive impairment (SCI) as the pre-mild cognitive
impairment (MCI) stage of subsequently manifest Alzheimer’s
disease. Int Psychogeriatr 2008;20(1):1-16.
273. Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E,
Kaduszkiewicz H, et al. AD dementia risk in late MCI, in early
MCI, and in subjective memory impairment. Alzheimers Dement
2014;10(1):76-83.
274. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M,
Chételat G, et al. A conceptual framework for research on
subjective cognitive decline in preclinical Alzheimer’s disease.
Alzheimers Dement 2014;10(6):844-52.
275. Buckley RF, Maruff P, Ames D, Bourgeat P, Martins RN, Masters
CL, et al. Subjective memory decline predicts greater rates of
clinical progression in preclinical Alzheimer's disease.
Alzheimers Dement 2016;12(7):796-804.
276. Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J,
et al. The source of cognitive complaints predicts diagnostic
conversion differentially among nondemented older adults.
Alzheimers Dement 2014;10(3):319-27.
277. Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K. Memory
complaints and risk of cognitive impairment after nearly 2
decades among older women. Neurology 2015;85(21):1852-8.
278. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome
over seven years of healthy adults with and without subjective
cognitive impairment. Alzheimers Dement 2010;6(1):11-24.
279. Fernandez-Blazquez MA, Avila-Villanueva M, Maestu F, Medina
M. Specific features of subjective cognitive decline predict
faster conversion to mild cognitive impairment. J Alzheimers
Dis 2016;52(1):271-81.
280. Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y,
Molinuevo JL, et al. The characterisation of subjective cognitive
decline. Lancet Neurol 2020;19(3):271-8.
281. Wolfsgruber S, Kleineidam L, Wagner M, Mösch E, Bickel H,
Lühmann D, et al. Differential risk of incident Alzheimer's
disease dementia in stable versus unstable patterns of subjective
cognitive decline. J Alzheimers Dis 2016;54(3):1135-46.
282. Unpublished data from the 2019-2020 Behavioral Risk Factor
Surveillance System survey conducted in 46 states and the
District of Columbia, analyzed and provided to the Alzheimer's
Association by the Alzheimer's Disease Program, Centers for
Disease Control and Prevention.
283. Dhana K, Beck T, Desai P, Wilson RS, Evans DA, Rajan KB.
Prevalence of Alzheimer's disease dementia in the 50 US states
and 3142 counties: A population estimate using the 2020
bridged-race postcensal from the National Center for Health
Statistics. Alz Dement 2023;19(10):4388-95.
284. Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA.
Prevalence and incidence of clinically diagnosed Alzheimer's
disease dementia from 1994 to 2012 in a population study.
Alzheimers Dement 2019;15(1):1-7.
285. Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence
of Alzheimer disease in the United States projected to the
years 2000 through 2050. Alzheimer Dis Assoc Disord
2001;15(4):169-73.
286. Chene G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al.
Gender and incidence of dementia in the Framingham Heart
Study from mid-adult life. Alzheimers Dement 2015;11(3):
310-20.
287. U.S. Census Bureau. 2014 National Population Projections:
Downloadable Files. Available at: https://www.census.gov/data/
datasets/2014/demo/popproj/2014-popproj.html. Accessed
December 15, 2023.
288. Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al.
Lifetime risk of dementia and Alzheimer’s disease. The impact
of mortality on risk estimates in the Framingham Study.
Neurology 1997;49(6):1498-504.
289. Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is
the risk of developing Alzheimer’s disease greater for women
than for men? Am J Epidemiol 2001;153(2):132-6.
290. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A.
Age-specific incidence rates of Alzheimer's disease: The
Baltimore Longitudinal Study of Aging. Neurology
2000;54(11):2072-7.
291. Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ.
Bilingualism does not alter cognitive decline or dementia risk
among Spanish-speaking immigrants. Neuropsychology
2014;28(2):238-46.
292. Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz
A, Winblad B. Very old women at highest risk of dementia and
Alzheimer’s disease: Incidence data from the Kungsholmen
Project, Stockholm. Neurology 1997;48:132-8.
293. Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo
JM, Dartigues JF. Are sex and educational level independent
predictors of dementia and Alzheimer’s disease? Incidence data
from the PAQUID project. J Neurol Neurosurg Psychiatry
1999;66:177-83.
294. Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE,
Arthur A, et al. A two decade dementia incidence comparison
from the Cognitive Function and Ageing Studies I and II. Nat
Commun 2016;7:11398.
295. Mielke MM, Ferretti MT, Iulita MF, Hayden K, Khachaturian AS.
Sex and gender in Alzheimer's disease — Does it matter?
Alzheimers Dement 2018;14(9):1101-1103.
296. Rocca WA. Time, Sex, gender, history, and dementia. Alzheimer
Dis Assoc Disord 2017;31(1):76-9.
297. Shaw C, Hayes-Larson E, Glymour MM, Dufouil C, Hohman TJ,
Whitmer RA. Evaluation of selective survival and sex/gender
differences in dementia incidence using a simulation model.
JAMA Netw Open 2021;4(3):e211001.
298. Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP, Jr.,
Whitmer RA. Reproductive period and risk of dementia in a
diverse cohort of health care members. Neurology
2019;92(17):e2005-e2014.
299. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of
Alzheimer's disease: Assessing sex and gender differences. Clin
Epidemiol 2014;6:37-48.
300. Mielke MM, Aggarwal NT, Vila-Castelar C, Agarwal P, Arenaza-
Urquijo EM, Brett B, et al. Consideration of sex and gender in
Alzheimer's disease and related disorders from a global
perspective. Alzheimers Dement 2022;10.1002/alz.12662.
301. Rocca WA, Mielke MM, Vemuri P, Miller VM. Sex and gender
differences in the causes of dementia: A narrative review.
Maturitas 2014;79(2):196-201.
302. Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA,
Kabeto MU, et al. A comparison of the prevalence of dementia
in the United States in 2000 and 2012. JAMA Intern Med
2017;177(1):51-8.
303. Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A,
Amaducci LA, et al. Rates and risk factors for dementia and
Alzheimer's disease: Results from EURODEM pooled analyses.
EURODEM Incidence Research Group and Work Groups.
European Studies of Dementia. Neurology 1999;52(1):78-84.
126 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
304. Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty
GD. Socioeconomic status as a risk factor for dementia death:
individual participant meta-analysis of 86 508 men and women
from the UK. Br J Psychiatry 2013;203(1):10-7.
305. Mayeda RM, Mobley TM, Weiss RE, Murchland AR, Berkman LF,
Sabbath EL. Association of work-family experience with mid-
and late-life memory decline in US women. Neurology 2020;
e3072-33080.
306. Mielke MM, James BD. Women who participated in the paid
labor force have lower rates of memory decline: Working to
remember. Neurolgy 2020;95(23):1027-8.
307. Population Reference Bureau. Women, Work, and the COVID
Pandemic: Myths and Realities. Available at: https://www.prb.
org/articles/blog-u-s-women-work-and-the-covid-pandemic-
myths-and-realities. Accessed December 15, 2023.
308. Zamarro G, Prados MJ. “Gender Differences in Couples’
Division of Childcare, Work, and Mental Health During
COVID-19,” Rev Econ Househ 2021;19(1):11-40.
309. Center for American Progress. Calculating the Hidden Cost of
Interrupting a Career for Child Care. Available at: https://www.
americanprogress.org/article/calculating-the-hidden-cost-of-
interrupting-a-career-for-child-care/. Accessed December 15, 2023.
310. Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and
gender differences in Alzheimer’s disease: Recommendations
for future research. J Womens Health 2012;21(10):1018-23.
311. Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s
Disease Neuroimaging Initiative Investigators. Sex modifies the
APOE-related risk of developing Alzheimer disease. Ann Neurol
2014;75(4):563-73.
312. Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender,
and Alzheimer’s disease: An overlooked, but potent and
promising interaction. Brain Imaging Behav 2014;8(2):262-73.
313. Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M,
Beecham G, Kunkle B, et al. Sex-specific association of
apolipoprotein E with cerebrospinal fluid levels of tau. JAMA
Neurol 2018;75(8):989-98.
314. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et
al. Apolipoprotein E genotype and sex risk factors for
Alzheimer disease: A meta-analysis. JAMA Neurol
2017;74(10):1178-89.
315. Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use,
APOE, and cognitive decline: Evidence of gene-environment
interaction. Neurology 2000;54(10):1949-54.
316. Kang JH, Grodstein F. Postmenopausal hormone therapy,
timing of initiation, APOE and cognitive decline. Neurobiol
Aging 2012;33(7):1129-37.
317. Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS,
Fazio S. Diagnosis and assessment of Alzheimer’s disease in
diverse populations. Alzheimers Dement 2008;4(4):305-9.
318. Manly JJ, Mayeux R. Ethnic differences in dementia and
Alzheimer’s disease. In: Anderson N, Bulatao R, Cohen B, eds.
Critical perspectives on racial and ethnic differentials in health
in late life. Washington, D.C.: National Academies Press; 2004:
p. 95-141.
319. Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R,
Sevush S, et al. Prevalence of dementia in three ethnic groups:
The South Florida Program on Aging and Health. Ann
Epidemiol 2003;13(6):472-78.
320. Harwood DG, Ownby RL. Ethnicity and dementia. Curr Psych
Report 2000;2(1):40-5.
321. Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon
SW. Incidence and prevalence of dementia in a multiethnic
cohort of municipal retirees. Neurology 1997;49(1):44-50.
322. Steenland K, Goldstein FC, Levey A, Wharton W. A meta-
analysis of Alzheimer's disease incidence and prevalence
comparing African-Americans and caucasians. J Alzheimers Dis
2015;50(1):71-6.
323. Power MC, Bennett EE, Turner RW, Dowling NM, Ciarleglio A,
Glymour MM, et al. Trends in relative incidence and prevalence
of dementia across non-Hispanic black and white individuals in
the United States, 2000-2016. JAMA Neurology
2021;78(3):275-84.
324. Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM,
Llewellyn DJ, et al. Cognitive performance and informant
reports in the diagnosis of cognitive impairment and dementia
in African Americans and whites. Alzheimers Dement
2009;5(6):445-53.
325. Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer
EH, et al. Rates of dementia in three ethnoracial groups. Int J
Geriatr Psychiatry 1999;14(6):481-93.
326. Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A,
Jagust WJ. Prevalence of dementia in older latinos: The
influence of type 2 diabetes mellitus, stroke and genetic
factors. J Am Geriatr Soc 2003;51:169-77.
327. Samper-Ternent R, Kuo YF, Ray LA, Ottenbacher KJ, Markides
KS, Al Snih S. Prevalence of health conditions and predictors
of mortality in oldest old Mexican Americans and non-Hispanic
whites. J Am Med Dir Assn 2012;13(3):254-9.
328. Mehta KM, Yeo GW. Systematic review of dementia prevalence
and incidence in United States race/ethnic populations.
Alzheimers Dement 2017;13(1):72-83.
329. González HM, Tarraf W, Schneiderman N, Fornage M, Vásquez
PM, Zeng D, et al. Prevalence and correlates of mild cognitive
impairment among diverse Hispanics/Latinos: Study of
Latinos-Investigation of Neurocognitive Aging results.
Alzheimers Dement 2019;15(12):1507-15.
330. Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster
A, et al. Effect of socioeconomic disparities on incidence of
dementia among biracial older adults: Prospective study. BMJ
2013;347:f7051.
331. Kornblith E, Bahorik A; Boscardin WJ; Xia F, Barnes DE, Yaffe
K. Association of Race and Ethnicity With Incidence of
Dementia Among Older Adults. JAMA 2022;327(15):1488-95.
332. Chin AL, Negash S, Hamilton R. Diversity and disparity in
dementia: The impact of ethnoracial differences in Alzheimer
disease. Alzheimer Dis Assoc Disord 2011;25(3):187-95.
333. Froehlich TE, Bogardus Jr. ST, Inouye SK. Dementia and race:
Are there differences between African Americans and
Caucasians? J Am Geriatr Soc 2001;49(4):477-84.
334. Glymour MM, Manly JJ. Lifecourse social conditions and racial
and ethnic patterns of cognitive aging. Neuropsychol Rev
2008;18(3):223-54.
335. Bailey ZD, Feldman JM, Bassett MT. How Structural Racism
Works - Racist Policies as a Root Cause of U.S. Racial Health
Inequities. N Engl J Med 2021;384(8):768-73.
336. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT.
Structural racism and health inequities in the USA: Evidence
and interventions. Lancet 2017;389(10077):1453-63.
337. Caunca MR, Odden MC, Glymour MM, Elfassy T, Kershaw KN,
Sidney S, et al. Association of racial residential segregation
throughout young adulthood and cognitive performance in
middle-aged participants in the CARDIA study. JAMA
Neurology 2020;77(8):1000-7.
338. Lamar M, Lerner AJ, James BD, Yu L, Glover CM, Wilson RS,
et al. Relationship of early-life residence and educational
experience to level and change in cognitive functioning:
Results of the Minority Aging Research Study. J Gerontol B
Psychol Sci Soc Sci 2020;75(7):e81-e92.
339. Peterson RL, George KM, Barnes LL, Gilsanz P, Mayeda ER,
Glymour MM, Mungas D, Whitmer RA. Timing of School
Desegregation and Late-Life Cognition in the Study of
Healthy Aging in African Americans (STAR). JAMA Netw Open
2021;4(10):e2129052.
340. Bancks MP, Byrd GS, Caban-Holt A, Fitzpatrick AL, Forrester
SN, Hayden KM, et al. Self-reported experiences of
discrimination and incident dementia. Alzheimers Dement
2023;19(7):3119-28.
341. Lines LM, Sherif NA, Wiener JM. Racial and ethnic disparities
among individuals with Alzheimer’s disease in the United
States: A literature review. Research Triangle Park, NC: RTI
Press; 2014.
342. Zhang Z, Hayward MD, Yu YL. Life course pathways to racial
disparities in cognitive impairment among older Americans. J
Health Soc Behav 2016;57(2):184-99.
127
Appendices
343. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR,
Ofstedal MB, et al. Prevalence of dementia in the United States:
The Aging, Demographics, and Memory Study.
Neuroepidemiology 2007;29(1-2):125-32.
344. Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner
V, Zhang R, et al. Impediments to timely diagnosis of
Alzheimer’s disease in African Americans. J Am Geriatr Soc
2005;53(11):2012-7.
345. Fitten LJ, Ortiz F, Ponton M. Frequency of Alzheimer’s disease
and other dementias in a community outreach sample of
Hispanics. J Am Geriatr Soc 2001;49(10):1301-8.
346. Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D,
et al. Racial and ethnic estimates of Alzheimer's disease and
related dementias in the United States (2015-2060) in adults
aged ≥ 65 years. Alzheimers Dement 2019;15(1):17-24.
347. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA.
Inequalities in dementia incidence between six racial and ethnic
groups over 14 years. Alzheimers Dement 2016;12(3):216-24.
348. Mayeda ER, Glymour MM, Quesenberry CP, Jr., Whitmer RA.
Heterogeneity in 14-year dementia incidence between Asian
American subgroups. Alzheimer Dis Assoc Disord
2017;31(3):181-6.
349. Ajrouch KJ, Zahodne LB, Antonucci TC. Arab American
Cognitive Aging: Opportunities for Advancing Research on
Alzheimer’s Disease Disparities. Innov Aging 2017;1(3):igx034.
350. Flatt JD, Cicero EC, Lambrou NH, Wharton W, Anderson JG,
Bouldin ED, McGuire LC, Taylor CA. Subjective cognitive
decline higher among sexual and gender minorities in the
United States, 2015-2018. Alzheimers dement
2021;7(1):e12197.
351. Liu H, Hsieh N, Zhang Z, Zhang Y, Langa KM. Same-sex couples
and cognitive impairment: Evidence from the health and
retirement study. J Gerontol B 2021;76(7):1388-99.
352. Hsieh N, Liu H, Lai WH. Elevated risk of cognitive impairment
among older sexual minorities: Do health conditions, health
behaviors, and social connections matter? Gerontologist
2021;61(3):352-62.
353. Perales-Puchalt J, Gauthreaux K, Flatt J, Teylan MA, Resendez
J, Kukull WA, Chan KCG, Burns J, Vidoni ED. Risk of dementia
and mild cognitive impairment among older adults in same-sex
relationships. Int J Geriatr Psychiatry 2019;34(6):828-35.
354. Dragon CN, Guerino P, Ewald E, Laffan AM. Transgender
Medicare beneficiaries and chronic conditions: exploring
fee-for-service claims data. LGBT health 2017;4(6):404-11.
355. Guo Y, Li Q, Yang X, Jaffee MS, Wu Y, Wang F, Bian J.
Prevalence of Alzheimer's and Related Dementia Diseases and
Risk Factors Among Transgender Adults, Florida, 20122020.
Am J Public Health 2022;112(5):754-7.
356. Romanelli RJ, Rosenblatt AS, Marcum ZA, Flatt JD. Cognitive
Impairment in Sexual and Gender Minority Groups: A Scoping
Review of the Literature. LGBT Health 2023. doi: 10.1089/
lgbt.2023.0095.
357. Meyer IH. Prejudice, social stress, and mental health in lesbian,
gay, and bisexual populations: conceptual issues and research
evidence. Psychol Bull 2003;129(5):674-97.
358. Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, Brennan-
Ing M. Correlates of Subjective Cognitive Decline in Lesbian,
Gay, Bisexual, and Transgender Older Adults. J Alzheimers Dis
2018;64(1):91-102.
359. Brennan-Ing M, Seidel L, Larson B, Karpiak SE. Social care
networks and older LGBT adults: challenges for the future. J
Homosex 2014;61(1):21-52.
360. Cohen RA, Seider TR, Navia B: HIV effects on age-associated
neurocognitive dysfunction: premature cognitive aging or
neurodegenerative disease? Alzheimers Res Ther 2015;7:37.
361. Cicero EC, Lett E, Flatt JD, Benson GP, Epps F. Transgender
Adults From Minoritized Ethnoracial Groups in the U.S. Report
Greater Subjective Cognitive Decline. J Gerontol B Psychol Sci
Soc Sci 2023;78(6):1051-9.
362. Hill Collins, Patricia. Intersectionality as Critical Social Theory.
Duke University Press. 2019.
363. Correro AN, Nielson KA. A review of minority stress as a risk
factor for cognitive decline in lesbian, gay, bisexual, and
transgender (LGBT) elders. J Gay Lesbian Ment Health
2020;24(1):2-19.
364. Flatt JD, Cicero EC, Kittle KR, Brennan-Ing M.
Recommendations for advancing research with sexual and
gender minority older adults. J Gerontol B 2022;77(1):1-9.
365. Wolters FJ, Chibnik LB, Waziry R, Anderson R, Berr C, Beiser
A, et al. Twenty-seven-year time trends in dementia incidence
in Europe and the United States. The Alzheimer Cohorts
Consortium. Neurology 2020;95(5):e519-e531.
366. Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA,
Hall KS, et al. Trends in the incidence and prevalence of
Alzheimer's disease, dementia, and cognitive impairment in the
United States. Alzheimers Dement 2011;7(1):80-93.
367. Wu YT, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C,
Hendrie HC, et al. The changing prevalence and incidence of
dementia over time: Current evidence. Nat Rev Neurol
2017;13(6):327-39.
368. Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram
MA, Breteler MM. Is dementia incidence declining? Trends in
dementia incidence since 1990 in the Rotterdam Study.
Neurology 2012;78(19):1456-63.
369. Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L.
Twenty-year changes in dementia occurrence suggest
decreasing incidence in central Stockholm, Sweden. Neurology
2013;80(20):1888-94.
370. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C,
Seshadri S. Incidence of dementia over three decades in the
Framingham Heart Study. N Engl J Med 2016;374:523-32.
371. Cerasuolo JO, Cipriano LE, Sposato LA, Kapral MK, Fang J, Gill
SS, et al. Population-based stroke and dementia incidence
trends: Age and sex variations. Alzheimers Dement
2017;13(10):1081-8.
372. Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia
incidence in a birth cohort analysis of the Einstein Aging Study.
JAMA Neurol 2017;74(11):1345-51.
373. Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, Shipley MJ,
Muniz-Terrera G, Singh-Manoux A, et al. Temporal trend in
dementia incidence since 2002 and projections for prevalence
in England and Wales to 2040: Modelling study. BMJ
2017;358:j2856.
374. Sullivan KJ, Dodge HH, Hughes TF, Chang CH, Zhu X, Liu A,
et al. Declining incident dementia rates across four population-
based birth cohorts. J Gerontol A Biol Sci Med Sci
2019;74(9):1439-45.
375. Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C,
Robinson L, et al. A two-decade comparison of prevalence of
dementia in individuals aged 65 years and older from three
geographical areas of England: Results of the Cognitive
Function and Ageing Study I and II. Lancet
2013;382(9902):1405-12.
376. Wiberg P, Waern M, Billstedt E, Östling S, Skoog I. Secular
trends in the prevalence of dementia and depression in
Swedish septuagenarians 1976–2006. Psychol Med
2013;43:2627-34.
377. Wimo A, Sjölund BM, Sköldunger A, Qiu C, Klarin I, Nordberg
G, et al. Cohort effects in the prevalence and survival of
people with dementia in a rural area in Northern Sweden. J
Alzheimers Dis 2016;50:387-96.
378. Hall KS, Gao S, Baiyewu O, Lane KA, Gureje O, Shen J, et al.
Prevalence rates for dementia and Alzheimer’s disease in
African Americans: 1992 versus 2000. Alzheimers Dement
2009;5(3):227-33.
379. Farina MP, Zhang YS, Kim JK, Hayward MD, Crimmins EM.
Trends in dementia prevalence, incidence, and mortality in the
United States (2000-2016). J Aging Health 2022;34(1):100–8.
380. van den Kommer TN, Deeg DJH, van der Flier WM, and Comijs
HC. Time trend in persistent cognitive decline: Results from
the longitudinal aging study Amsterdam. J Gerontol B Psychol
Sci Soc Sci 2018;73(Suppl 1)S57-64.
128 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
381. Sekita A, Ninomiya T, Tanizaki Y, Doi Y, Hata J, Yonemoto K,
et al. Trends in prevalence of Alzheimer's disease and vascular
dementia in a Japanese community: The Hisayama Study. Acta
Psychiatr Scand 2010;122(4):319-25.
382. Gao S, Burney HN, Callahan CM, Purnell CE, Hendrie HC.
Incidence of Dementia and Alzheimer Disease Over Time: A
Meta-Analysis. J Am Geriatr Soc. Jul 2019;67(7):1361-9.
383. Crimmins EM, Saito Y, Kim JK, Zhang Y, Sasson I, Hayward MD.
Educational differences in the prevalence of dementia and life
expectancy with dementia in the United States: Changes from
2000 to 2010. J Gerontol B Psychol Sci Soc Sci 2018;73(Suppl
1):S20-28.
384. Choi H, Schoeni RF, Martin LG, Langa K M. Trends in the
prevalence and disparity in cognitive limitations of Americans
55-69 years old. J Gerontol B Psychol Sci Soc Sci 2018;73
(Suppl 1):S29-37.
385. Zheng H. A New Look at Cohort Trend and Underlying
Mechanisms in Cognitive Functioning. J Gerontol B Psychol Sci
Soc Sci 2021;76(8):1652-63.
386. Freedman VA, Kasper JD, Spillman BC, Plassman BL. Short-
term changes in the prevalence of probable dementia: An
analysis of the 2011–2015 National Health and Aging Trends
Study. J Gerontol B Psychol Sci Soc Sci 2018;73(Suppl 1)
S48-56.
387. Langa KM. Is the risk of Alzheimer's disease and dementia
declining? Alzheimers Res Ther 2015;7(1):34.
388. Larson EB, Yaffe K, Langa KM. New insights into the dementia
epidemic. N Engl J Med 2013;369(24):2275-7.
389. Sheffield KM, Peek MK. Changes in the prevalence of cognitive
impairment among older Americans, 1993-2004: Overall
trends and differences by race/ethnicity. Am J Epidemiol
2011;174(3):274-83.
390. Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular
trends in cognitive performance in older black and white U.S.
adults, 1993-2012: Findings from the Chicago Health and
Aging Project. J Gerontol B Psychol Sci Soc Sci 2018;73
(Suppl 1):S73-81.
391. Prince MJ, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M.
World Alzheimer Report 2015: The Global Impact of Dementia:
An Analysis of Prevalence, Incidence, Cost and Trends; 2015.
392. de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS,
Seshadri S. The chronic neuropsychiatric sequelae of
COVID-19: The need for a prospective study of viral impact on
brain functioning. Alzheimers Dement 2021;17(6):1056-65.
393. Arias E, Tejada-Vera B, Kochanek KD, Ahmad FB. Provisional
Life Expectancy Estimates for 2021. National Vital Statistics
System, Report No. 23, 2022. Available at: https://www.cdc.
gov/nchs/data/vsrr/vsrr023.pdf. Accessed December 15, 2023.
394. The World Bank. Fertility, total (births per woman)—US.
Available at: https://data.worldbank.org/indicator/SP.DYN.
TFRT.IN?locations=US. Accessed December 15, 2023.
395. U.S. Census Bureau. 2017 National Population Projections
Tables. Available at: https://www.census.gov/data/tables/2017/
demo/popproj/2017-summary-tables.html. Accessed
December 15, 2023.
396. Administration for Community Living. 2019 Profile of Older
Americans. May 2020 Available at: https://acl.gov/sites/default/
files/Aging%20and%20Disability%20in%20
America/2019ProfileOlderAmericans508.pdf. Accessed
December 15, 2023.
397. Bauman K. Shift Toward Greater Educational Attainment for
Women Began 20 Years Ago. U.S. Census Bureau. Available at:
https://www.census.gov/newsroom/blogs/random-
samplings/2016/03/shift-toward-greater-educational-
attainment-for-women-began-20-years-ago.html. Accessed
December 15, 2023.
398. Population Reference Bureau. Why is the U.S. Birth Rate
Declining? 2021. Available at: https://www.prb.org/resources/
why-is-the-u-s-birth-rate-declining/. Accessed December 15, 2023.
399. Horowitz JM, Igielnik R, Kochhar R. Trends in income and
wealth inequality. Pew Research Center. Available at: https://
www.pewresearch.org/social-trends/2020/01/09/trends-in-
income-and-wealth-inequality/. Accessed October 28, 2023.
400. Tom SE, Phadke M, Hubbard RA, Crane PK, Stern Y, Larson EB.
Association of Demographic and Early-Life Socioeconomic
Factors by Birth Cohort with Dementia Incidence Among US
Adults Born Between 1893 and 1949. JAMA Netw Open
2020;3(7):e2011094.
401. Skoog I. Dementia incidence: The times, they are a-
changing. Nature Rev Neurol 2016;12:316-8. Available at:
https://www.nature.com/articles/nrneurol.2016.55. Accessed
December 15, 2023.
402. Sullivan KJ, Dodge HH, Hughes TF, Chang C-C, Zhu X, Liu A,
et al. Declining incident dementia rates across four
population-based birth cohorts. J Gerontol A Biol Sci Med Sci
2019;74(9):1439-45.
403. Centers for Disease Control and Prevention, National Center
for Health Statistics. National Vital Statistics System, Mortality
2018-2021 on CDC WONDER Online Database, released in
2021. Data are from the Multiple Cause of Death Files,
2018-2021, as compiled from data provided by the 57 vital
statistics jurisdictions through the Vital Statistics Cooperative
Program. Available at: http://wonder.cdc.gov/ucd-icd10-
expanded.html. Accessed December 13, 2023.
404. U.S. Department of Health and Human Services. Centers
for Disease Control and Prevention. National Center for
Health Statistics. CDC WONDER online database: About
Provisional Mortality Statistics, 2018 through Last Month.
https://wonder.cdc.gov/mcd-icd10-provisional.html. Accessed
December 15, 2023.
405. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany
E. The State of US Health, 1990-2016: Burden of Diseases,
Injuries, and Risk Factors Among US States. JAMA
2018;319(14):1444-72.
406. World Health Organization. International Statistical
Classification of Diseases and Related Health Problems. 10th
revision. 2nd edition. WHO Press: Geneva, Switzerland; 2004.
407. Burns A, Jacoby R, Luthert P, Levy R. Cause of death in
Alzheimer’s disease. Age Ageing 1990;19(5):341-4.
408. Brunnstrom HR, Englund EM. Cause of death in patients with
dementia disorders. Eur J Neurol 2009;16(4):488-92.
409. Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between
nosologist and Cardiovascular Health Study review of deaths:
Implications of coding differences. J Am Geriatr Soc
2009;57(1):133-9.
410. Romero JP, Benito-Leon J, Louis ED, Bermejo-Pareja F. Under
reporting of dementia deaths on death certificates: A
systematic review of population-based cohort studies. J
Alzheimers Dis 2014;41(1):213-21.
411. Ganguli M, Rodriguez EG. Reporting of dementia on death
certificates: A community study. J Am Geriatr Soc
1999;47(7):842-9.
412. Stokes AC, Weiss J, Lundberg DJ, Xie W, Kim JK, Preston SH,
et al. Estimates of the association of dementia with US
mortality levels using linked survey and mortality records.
JAMA Neurol 2020;77(12):1543-50.
413. Unpublished tabulations based on data from the 100% National
Sample Medicare Fee-for-Service Beneficiaries for 2019.
Prepared under contract by Health Care Cost Institute,
November 2021.
414. Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the
United States among persons with Alzheimer’s disease
(2010-2050). Alzheimers Dement 2014;10(2):E40-6.
415. Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ.
Lethality of Alzheimer disease and its impact on nursing home
placement. Alzheimer Dis Assoc Disord 2010;24(1):90-5.
416. Centers for Disease Control and Prevention. National Center
for Health Statistics. Excess Deaths Associated with COVID-19.
Available at: https://www.cdc.gov/nchs/nvss/vsrr/covid19/
excess_deaths.htm. Accessed December 15, 2023.
417. Gilstrap L, Zhou W, Alsan M, Nanda A, Skinner JS. Trends in
Mortality Rates Among Medicare Enrollees With Alzheimer
Disease and Related Dementias Before and During the Early
Phase of the COVID-19 Pandemic. JAMA Neurol
2022;79(4):342-8.
129
Appendices
418. Chen R, Charpignon M, Raquib RV, Wang J, Meza E, Aschmann
HE, et al. Excess Mortality With Alzheimer Disease and Related
Dementias as an Underlying or Contributing Cause During
the COVID-19 Pandemic in the US. JAMA Neurol
2023;80(9):919-28.
419. Tejada-Vera B. Mortality from Alzheimer’s disease in the United
States: Data for 2000 and 2010. National Center for Health
Statistics Data Brief, No. 116. National Center for Health
Statistics, Hyattsville, MD; 2013.
420. Taylor C, Greenlund S, McGuire L, Lu H, Croft J. Deaths from
Alzheimer’s Disease — United States, 1999-2014. MMWR
Morb Mortal Wkly Rep 2017;66:521-6.
421. Mitchell SL, Teno JM, Miller SC, Mor V. A national study of the
location of death for older persons with dementia. J Am Geriatr
Soc 2005;53(2):299-305.
422. U.S. Burden of Disease Collaborators, Mokdad AH, Ballestros K,
et al. The state of U.S. health, 1990-2016: Burden of diseases,
injuries, and risk factors among U.S. states. JAMA
2018;319(14):1444-72.
423. Barker C, Green A. Opening the debate on DALYs (disability-
adjusted life years). Health Policy Plan 1996;11(2):179-83.
424. Gaugler JE, Kane RL, Kane RA. Family care for older adults with
disabilities: Toward more targeted and interpretable research.
Int J Aging Hum Dev 2002;54(3):205-31.
425. Schulz R, Quittner AL. Caregiving through the life-span:
Overview and future directions. Health Psychol 1998;17:107-11.
426. Friedman EM, Shih RA, Langa KM, Hurd MD. U.S. prevalence
and predictors of informal caregiving for dementia. Health Aff
2015;34(10):1637-41.
427. Spillman B, Wolff J, Freedman VA, Kasper JD. Informal
Caregiving for Older Americans: An Analysis of the 2011
National Health and Aging Trends Study. Available at: https://
aspe.hhs.gov/pdf-report/informal-caregiving-older-americans-
analysis-2011-national-health-and-aging-trends-study.
Accessed December 15, 2023.
428. Walmart: 2023 Annual Report. Available at: https://s201.q4cdn.
com/262069030/files/doc_financials/2023/ar/Walmart-10K-
Reports-Optimized.pdf. Accessed December 15, 2023.
429. McDonald’s Corporation Report 2021. Available at: https://
corporate.mcdonalds.com/content/dam/sites/corp/nfl/pdf/
MCD%202021%20Annual%20Report.pdf. Accessed December
15, 2023.
430. Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B,
Kuntz KM. Societal and family lifetime cost of dementia:
Implications for policy. J Am Geriatr Soc 2017;65(10):2169-75.
431. Official Data Foundation. CPI inflation calculator. Available at:
http://www.in2013dollars.com/2017-dollars-in-
2018?amount=139765. Accessed December 15, 2023.
432. Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and
indirect cost of managing Alzheimer's disease and related
dementias in the United States. Expert Rev Pharmacoecon
Outcomes Res 2017;17(2):189-202.
433. Greenwood N, Smith R. Motivations for being informal carers
of people living with dementia: A systematic review of
qualitative literature. BMC Geriatr 2019;19(1):169.
434. Kasper JD, Freedman VA, Spillman BC, Wolff JL. The
disproportionate impact of dementia on family and unpaid
caregiving to older adults. Health Aff 2015;34(10):1642-49.
435. Ornstein KA, Wolff JL, Bollens-Lund E, Rahman OK, Kelley AS.
Spousal caregivers are caregiving alone in the last years of life.
Health Aff (Millwood) 2019;38(6):964-72.
436. Alzheimer's Association. Issues Brief: LGBT and Dementia.
Available at https://www.alz.org/media/Documents/lgbt-
dementia-issues-brief.pdf. Accessed December 15, 2023.
437. Fredriksen-Goldsen KI, Jen S, Bryan AEB, Goldsen J. Cognitive
impairment, Alzheimer’s disease, and other dementias in the
lives of lesbian, gay, bisexual and transgender (LGBT) older
adults and their caregivers: Needs and competencies. J Appl
Gerontol 2018;37(5):545-69.
438. Candrian C, Burke ES, Kline D, Torke AM. Experiences of
caregiving with Alzheimer's disease in the LGBT community.
BMC Geriatr 2023;23(1):293.
439. Anderson JG, Flatt JD, Jabson Tree JM, Gross AL, Rose KM.
Characteristics of Sexual and Gender Minority Caregivers of
People With Dementia. J Aging Health 2021;33(10):838-51.
440. Kasper JD, Freedman VA, Spillman BC. Disability and Care
Needs of Older Americans by Dementia Status: An Analysis of
the 2011 National Health and Aging Trends Study. U.S.
Department of Health and Human Services; 2014. Available at:
http://aspe.hhs.gov/report/disability-and-care-needs-older-
americans-dementia-status-analysis-2011-national-health-
and-aging-trends-study. Accessed December 15, 2023.
441. Rabarison KM, Bouldin ED, Bish CL, McGuire LC, Taylor CA,
Greenlund KJ. The economic value of informal caregiving for
persons with dementia: Results from 38 states, the District of
Columbia, and Puerto Rico, 2015 and 2016 BRFSS. Am J
Public Health 2018;108(10):1370-7.
442. National Alliance for Caregiving in Partnership with the
Alzheimer’s Association. Dementia Caregiving in the U.S.
Bethesda, MD. Available at: https://www.caregiving.org/
wp-content/uploads/2020/05/Dementia-Caregiving-in-the-
US_February-2017.pdf. Accessed December 15, 2023.
443. Unpublished data from the 2016-2022 Behavioral Risk Factor
Surveillance System survey, analyzed by and provided to the
Alzheimer's Association by the Alzheimer's Disease and
Healthy Aging Program (AD+HP), Centers for Disease Control
and Prevention (CDC).
444. Fisher GG, Franks MM, Plassman BL, Brown SL, Potter GG,
Llewellyn D, et al. Caring for individuals with dementia and
cognitive impairment, not dementia: Findings from The Aging,
Demographics, and Memory Study. J Am Geriatr Soc
2011;59(3):488-94.
445. Riffin C, Van Ness PH, Wolff JL, Fried T. Family and other
unpaid caregivers and older adults with and without dementia
and disability. J Am Geriatr Soc 2017;65(8):1821-8.
446. National Poll on Healthy Aging. Dementia Caregivers:
Juggling, Delaying and Looking Forward. Available at: http://
www.healthyagingpoll.org/sites/default/files/2017-10/
NPHA_Caregivers-Report-PROOF_101817_v2.pdf. Accessed
December 15, 2023.
447. Caregiving in the U.S.: 2020 Report. Available at: https://www.
aarp.org/content/dam/aarp/ppi/2020/05/full-report-
caregiving-in-the-united-states.doi.10.26419-
2Fppi.00103.001.pdf. Accessed December 15, 2023.
448. Ohno S, Chen Y, Sakamaki H, Matsumaru N, Yoshino M,
Tsukamoto K. Burden of caring for Alzheimer's disease or
dementia patients in Japan, the US, and EU: results from the
National Health and Wellness Survey: a cross-sectional survey.
J Med Econ 2021;24(1):266-78.
449. National Alliance for Caregiving and AARP. Caregiving in the
U.S.: Unpublished data analyzed under contract for the
Alzheimer’s Association; 2009.
450. Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and
Figures. Special Report: Women and Alzheimer’s Disease.
Available at: https://www.alzheimersanddementia.com/article/
S1552-5260(14)00062-4/fulltext. Accessed December 15, 2023.
451. Xiong C, Biscardi M, Astell A, Nalder E, Cameron JI, Mihailidis
A, et al. Sex and gender differences in caregiving burden
experienced by family caregivers of persons with dementia: A
systematic review. PLoS One 2020;15(4):e0231848.
452. Pinquart M, Sörensen. Gender differences in caregiver
stressors, social resources, and health: An updated meta-
analysis. J Gerontol B Psychol Sci Soc Sci 2006;61(1):P33-45.
453. Ma M, Dorstyn D, Ward L, Prentice S. Alzheimer's disease and
caregiving: A meta-analytic review comparing the mental
health of primary carers to controls. Aging Ment Health
2017;5:1-11.
454. Roberts HL, Bollens-Lund E, Ornstein KA, Kelley AS. Caring
for aging parents in the last years of life. J Am Geriatr Soc
2023;71(9):2871-7.
455. Fabius CD, Wolff JL, Kasper JD. Race differences in
characteristics and experiences of black and white caregivers
of older Americans. Gerontologist 2020;60(7):1244-53.
130 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
456. Moon HE, Rote SM, Sears J, Schepens Niemiec SL. Racial
Differences in the Dementia Caregiving Experience during the
COVID-19 Pandemic: Findings from the National Health and
Aging Trends Study (NHATS). J Gerontol B Psychol Sci Soc Sci
2022;gbac098.
457. Liu R, Chi I, Wu S. Caregiving Burden Among Caregivers of
People With Dementia Through the Lens of Intersectionality.
Gerontologist 2022;62(5):650-61.
458. Parker LJ, Fabius CD. Racial differences in respite use among
black and white caregivers for people living with dementia. J
Aging Health 2020;32(10):1667-75.
459. Ejem D, Atkins GC, Perkins M, Morhardt DJ, Williams IC,
Cothran FA. Stressors and Acceptability of Services Among
Black Caregivers of Persons With Memory Problems. J
Gerontol Nurs 2022;48(6):13-8.
460. Rote SM, Angel JL, Moon H, Markides K. Caregiving across
diverse populations: New evidence from the National Study of
Caregiving and Hispanic EPESE. Innov Aging 2019;3(2):igz033.
461. Pinquart M, Sörensen S. Ethnic differences in stressors,
resources, and psychological outcomes of family caregiving: A
meta-analysis. Gerontologist 2005;45(1):90-106.
462. Dilworth-Anderson P, Moon H, Aranda MP. Dementia
caregiving research: Expanding and reframing the lens of
diversity, inclusivity, and intersectionality. Gerontologist
2020;60(5):797-805.
463. Chen C, Thunell J, Zissimopoulos J. Changes in physical and
mental health of Black, Hispanic, and White caregivers and
non-caregivers associated with onset of spousal dementia.
Alzheimers Dement (N Y) 2020;6(1):e12082.
464. Cothran FA, Chang E, Beckett L, Bidwell JT, Price CA,
Gallagher-Thompson D. A Landscape of Subjective and
Objective Stress in African-American Dementia Family
Caregivers. West J Nurs Res 2022;44(3):239-49.
465. Liu C, Badana ANS, Burgdorf J, Fabius CD, Roth DL, Haley WE.
Systematic review and meta-analysis of racial and ethnic
differences in dementia caregivers' well-being. Gerontologist
2021;61(5):e228-e243.
466. Baik D, Centi S, McNair B. Assessing racial and ethnic
differences in cardiovascular disease in U.S. family caregivers of
persons with dementia: Analysis of data from the 2015-2020
Behavioral Risk Factor Surveillance System. Res Gerontol Nurs
2023;16(5):241-9.
467. Brewster GS, Bonds K, McLennon S, Moss KO, Epps F, Lopez
RP. Missing the Mark: The Complexity of African American
Dementia Family Caregiving. J Fam Nurs 2020;26(4):294-301.
468. Lewis JP, Manson SM, Jernigan VB, Noonan C. "Making Sense
of a Disease That Makes No Sense": Understanding Alzheimer's
Disease and Related Disorders Among Caregivers and
Providers Within Alaska Native Communities. Gerontologist
2021;61(3):363-73.
469. Parker LJ, Fabius C. Who's Helping Whom? Examination of
care arrangements for racially and ethnically diverse people
living with dementia in the community. J Appl Gerontol
2022;41(12):2589-93.
470. Idorenyin Imoh U, Charity T. Cultural and Social Factors in Care
Delivery Among African American Caregivers of Persons With
Dementia: A Scoping Review. Gerontol Geriatr Med
2023;9:23337214231152002.
471. Ta Park VM, Ly Q, von Oppenfeld J, Lee Y, Joo Y, Shin H-W,
et al. A scoping review of dementia caregiving for Korean
Americans and recommendations for future research. Clin
Gerontol 2023;46(2):223-39.
472. Moraes Balbim G, Magallanes M, Marques IG, Ciruelas K,
Aguiñaga S, Guzman J, et al. Sources of caregiving burden in
middle-aged and older Latino caregivers. J Geriatr Psychiatry
Neurol 2020;33(4):185-94.
473. Jaldin MA, Balbim GM, Colin SJ, Marques IG, Mejia J,
Magallanes M, et al. The influence of Latino cultural values on
the perceived caregiver role of family members with
Alzheimer's disease and related dementias. Ethn Health
2023;28(4):619-33.
474. Martinez IL, Gonzalez EA, Quintero C, Vania MJ. The
Experience of Alzheimer's Disease Family Caregivers in a Latino
Community: Expectations and incongruences in support
services. J Gerontol B Psychol Sci Soc Sci 2022;77(6):1083-93.
475. Sloan DH, Johnston D, Fabius C, Pyatt T, Antonsdottir I,
Reuland M. Transcending inequities in dementia care in Black
communities: Lessons from the maximizing independence at
home care coordination program. Dementia (London)
2022;21(5):1653-68.
476. Rote SM, Moon HE, Kacmar AM, Moore S. Exploring Coping
Strategies and Barriers in Dementia Care: A Mixed-Methods
Study of African American Family Caregivers in Kentucky. J
Appl Gerontol 2022;41(8):1851-9.
477. Alexander K, Oliver S, Bennett SG, Henry J, Hepburn K,
Clevenger C. "Falling between the cracks": Experiences of Black
dementia caregivers navigating U.S. health systems. J Am
Geriatr Soc 2022;70(2):592-600.
478. Bonner GJ, Freels S, Ferrans C, Steffen A, Suarez ML, Dancy
BL, et al. Advance Care Planning for African American
Caregivers of Relatives With Dementias: Cluster Randomized
Controlled Trial. Am J Hosp Palliat Care 2021;38(6):547-56.
479. Meyer OL, Sun M, Do T, Ho JN, Dinh B-T, Nguyen S, et al.
Community-Engaged Research with Vietnamese Americans to
Pilot-Test a Dementia Caregiver Intervention. J Cross Cult
Gerontol 2020;35(4):479-92.
480. Fields NL, Xu L, Richardson VE, Parekh R, Ivey D, Calhoun M.
Utilizing the Senior Companion Program as a platform for a
culturally informed caregiver intervention: Results from a mixed
methods pilot study. Dementia (London) 2021;20(1):161-87.
481. Guest MA, Smith MP. In Our Community, Dementia Speaks:
Pilot of a person-centered training targeting African-American
caregivers of persons-living with dementia (innovative
practice). Dementia (London) 2021;20(1):391-7.
482. Withers M, Cortez-Sanchez K, Herrera J, Ringman JM,
Segal-Gidan F. "My backpack is so heavy": Experiences of Latino
caregivers of family with early-onset Alzheimer's. J Am Geriatr
Soc. 2021;69(6):1539-47.
483. Epps F, Heidbreder V, Alexander K, Tomlinson A, Freeman V,
Williams N. A dementia-friendly church: How can the African
American church support families affected by dementia?
Dementia (London) 2021;20(2):556-69.
484. Park VT, Grill JD, Zhu J, Nguyen K, Nam B, Tsoh J, et al. Asian
Americans and Pacific Islanders' perspectives on participating in
the CARE recruitment research registry for Alzheimer's disease
and related dementias, aging, and caregiving research.
Alzheimers Dement (N Y) 2021;7(1):e12195.
485. Portacolone E, Palmer NR, Lichtenberg P, Waters CM, Hill CV,
Keiser S, et al. Earning the Trust of African American
Communities to Increase Representation in Dementia
Research. Ethn Dis 2020;30(Suppl 2):719-34.
486. Liu J, Lou Y, Wu B, Mui A. "I've been always strong to conquer
any suffering:" challenges and resilience of Chinese American
dementia caregivers in a life course perspective. Aging Ment
Health 2021;25(9):1716-24.
487. Bonds K, Song MK, Whitlatch CJ, Lyons KS, Kaye JA, Lee CS.
Patterns of Dyadic Appraisal of Decision-Making Involvement
of African American Persons Living With Dementia.
Gerontologist 2021;61(3):383-91.
488. Epps F, Alexander K, Brewster GS, Parker LJ, Chester M,
Tomlinson A, et al. Promoting dementia awareness in
African-American faith communities. Public Health Nurs
2020;37(5):715-21.
489. Racine L, Ford H, Johnson L, Fowler-Kerry S. An integrative
review of Indigenous informal caregiving in the context of
dementia care. J Adv Nurs 2022;78(4):895-917.
490. Epps F, Moore M, Chester M, Gore J, Sainz M, Adkins A, et al.
The Alter Program: A nurse-led, dementia-friendly program for
African American faith communities and families living with
dementia. Nurs Adm Q 2021;46(1):72-80.
491. Freedman VA, Patterson SE, Cornman JC, Wolff JL. A day in
the life of caregivers to older adults with and without dementia:
Comparisons of care time and emotional health. Alzheimers
Dement 2022;18(9):1650-61.
131
Appendices
492. National Alliance for Caregiving and AARP. Caregiving in the
U.S. (2015 Report). Available at: https://www.aarp.org/content/
dam/aarp/ppi/2015/caregiving-in-the-united-states-2015-
report-revised.pdf. Accessed December 15, 2023.
493. Spillman BC, Freedman VA, Kasper JD, Wolff JL. Change over
time in caregiving networks for older adults with and without
dementia. J Gerontol B Psychol Sci Soc Sci 2020;75(7):1563-72.
494. Port CL, Zimmerman S, Williams CS, Dobbs D, Preisser JS,
Williams SW. Families filling the gap: Comparing family
involvement for assisted living and nursing home residents with
dementia. Gerontologist 2005;45(Special Issue 1):87-95.
495. Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S.
Long-term care placement of dementia patients and caregiver
health and well-being. JAMA 2004;292(8):961-7.
496. Rattinger GB, Schwartz S, Mullins CD, Corcoran C, Zuckerman
IH, Sanders C, et al. Dementia severity and the longitudinal
costs of informal care in the Cache County population.
Alzheimers Dement 2015;11(8):946-54.
497. Rattinger GB, Fauth EB, Behrens S, Sanders C, Schwartz S,
Norton MC, et al. Closer caregiver and care-recipient
relationships predict lower informal costs of dementia care:
The Cache County Dementia Progression Study. Alzheimers
Dement 2016;12(8):917-24.
498. Song M-K, Paul S, Happ MB, Lea J, Pirkle JL, Jr., Turberville-
Trujillo L. Informal Caregiving Networks of Older Adults With
Dementia Superimposed on Multimorbidity: A Social Network
Analysis Study. Innov Aging 2023;7(4):igad033.
499. Wolff JL, Mulcahy J, Huang J, Roth DL, Covinsky K, Kasper JD.
Family Caregivers of Older Adults, 1999-2015: Trends in
characteristics, circumstances, and role-related appraisal.
Gerontologist 2018;58(6):1021-32.
500. Jutkowitz E, Gaugler JE, Trivedi AN, Mitchell LL, Gozalo P.
Family caregiving in the community up to 8-years after onset
of dementia. BMC Geriatr 2020;20(1):216.
501. Gaugler JE, Zarit SH, Pearlin LI. The onset of dementia
caregiving and its longitudinal implications. Psychol Aging
2003;18(2):171-80.
502. Jutkowitz E, Gozalo P, Trivedi A, Mitchell L, Gaugler JE. The
effect of physical and cognitive impairments on caregiving. Med
Care 2020;58(7):601-9.
503. Lei L, Maust DT, Leggett AN. Functional decline over time and
change in family and other unpaid care provided to community-
dwelling older adults living with and without dementia. J
Gerontol B Psychol Sci Soc Sci 2023;78(10):1727-34.
504. Ornstein K, Gaugler JE. The problem with “problem behaviors”:
A systematic review of the association between individual
patient behavioral and psychological symptoms and caregiver
depression and burden within the dementia patient-caregiver
dyad. Int Psychogeriatr 2012;24(10):1536-52.
505. Vaingankar JA, Chong SA, Abdin E, Picco L, Shafie S, Seow E,
et al. Psychiatric morbidity and its correlates among informal
caregivers of older adults. Compr Psychiatry 2016;68:178-85.
506. Feast A, Moniz-Cook E, Stoner C, Charlesworth G, Orrell M. A
systematic review of the relationship between behavioral and
psychological symptoms (BPSD) and caregiver well-being. Int
Psychogeriatr 2016;28(11):1761-74.
507. Schulz R, Beach SR. Caregiving as a risk factor for mortality:
The Caregiver Health Effects Study. JAMA 1999;282:2215-60.
508. Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to
one’s physical health? A meta-analysis. Psychol Bull
2003;129(6):946-72.
509. Liu W, Gallagher-Thompson D. Impact of dementia caregiving:
Risks, strains, and growth. In: Qualls SH, Zarit SH, eds. Aging
families and caregiving. Hoboken, NJ: John Wiley & Sons, Inc.;
2009: p. 85-112.
510. Pinquart M, Sörensen S. Associations of stressors and uplifts of
caregiving with caregiver burden and depressive mood: A
meta-analysis. J Gerontol B Psychol Sci Soc Sci
2003;58(2):112-28.
511. Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care:
Mental health effects, intervention strategies, and clinical
implications. Lancet Neurol 2006;5(11):961-73.
512. Goren A, Montgomery W, Kahle-Wrobleski K, Nakamura T,
Ueda K. Impact of caring for persons with Alzheimer’s disease
or dementia on caregivers’ health outcomes: Findings from a
community based survey in Japan. BMC Geriatr 2016;16:122.
513. Alzheimer’s Association. 2016 Alzheimer’s Disease Facts and
Figures. Alzheimer Dement 2016;12(4):459-509.
514. Jones RW, Lebrec J, Kahle-Wrobleski K, Dell'Agnello G, Bruno
G, Vellas B, et al. Disease progression in mild dementia due to
Alzheimer disease in an 18-month observational study
(GERAS): The impact on costs and caregiver outcomes.
Dement Geriatr Cogn Dis Extra 2017;20;7(1):87-100.
515. Leggett AN, Meyer OL, Bugajsky BC, Polenick CA. Accentuate
the Positive: The association between informal and formal
supports and caregiving gains. J Appl Gerontol
2021l;40(7):763-71.
516. Quinn C, Toms G. Influence of positive aspects of dementia
caregiving on caregivers' well-being: A systematic review.
Gerontologist 2019;59(5):e584-e596.
517. Zarit SH. Positive aspects of caregiving: More than looking on
the bright side. Aging Ment Health 2012;16(6):673-74.
518. Cheng ST, Mak EP, Lau RW, Ng NS, Lam LC. Voices of
Alzheimer caregivers on positive aspects of caregiving.
Gerontologist 2016;56(3):451-60.
519. Monin JK, Schulz R, Feeney BC. Compassionate love in
individuals with Alzheimer's disease and their spousal
caregivers: Associations with caregivers' psychological health.
Gerontologist 2015;55(6):981-9.
520. Roth DL, Dilworth-Anderson P, Huang J, Gross AL, Gitlin LN.
Positive aspects of family caregiving for dementia: Differential
item functioning by race. J Gerontol B Psychol Sci Soc Sci
2015;70(6):813-9.
521. Lloyd J, Patterson T, Muers J. The positive aspects of
caregiving in dementia: A critical review of the qualitative
literature. Dementia (London) 2016;15(6):1534-61.
522. Yu DSF, Cheng ST, Wang J. Unravelling positive aspects of
caregiving in dementia: An integrative review of research
literature. Int J Nurs Stud 2018;79:1-26.
523. Cheng ST. Positive aspects of caregiving attenuate the
relationship between behavioral bother and anxiety and
depressive symptoms in dementia family caregivers. Geriatr
Gerontol Int 2023;23(5):366-70.
524. Wennberg AM, Anderson LR, Cagnin A, Chen-Edinboro LP,
Pini L. How both positive and burdensome caregiver
experiences are associated with care recipient cognitive
performance: Evidence from the National Health and Aging
Trends Study and National Study of Caregiving. Front Public
Health. 2023;11:1130099.
525. van den Kieboom R, Snaphaan L, Mark R, Bongers I. The
trajectory of caregiver burden and risk factors in dementia
progression: A systematic review. J Alzheimers Dis
2020;77(3):1107-15.
526. Polenick CA, Min L, Kales HC. Medical Comorbidities of
dementia: Links to caregivers' emotional difficulties and gains.
J Am Geriatr Soc 2020;68(3):609-13.
527. Sheehan OC, Haley WE, Howard VJ, Huang J, Rhodes JD,
Roth DL. Stress, Burden, and Well-Being in Dementia and
Nondementia Caregivers: Insights From the Caregiving
Transitions Study. Gerontologist 2021;61(5):670-9.
528. Sallim AB, Sayampanathan AA, Cuttilan A, Chun-Man Ho R.
Prevalence of mental health disorders among caregivers of
patients with Alzheimer disease. J Am Med Dir Assoc
2015;16(12):1034-41.
529. Thunyadee C, Sitthimongkol Y, Sangon S, Chai-Aroon T,
Hegadoren KM. Predictors of depressive symptoms and
physical health in caregivers of individuals with schizophrenia.
J Nurs Health Sci 2015;17:412-9.
530. Harris ML, Titler MG, Hoffman GJ. Associations between
Alzheimer's disease and related dementias and depressive
symptoms of partner caregivers. J Appl Gerontol
2021;40(7):772-80.
531. Vitaliano PP, Ustundag O, Borson S. Objective and subjective
cognitive problems among caregivers and matched non-
caregivers. Gerontologist 2017;57(4):637-47.
132 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
532. Dassel KB, Carr DC, Vitaliano P. Does caring for a spouse with
dementia accelerate cognitive decline? Findings from the Health
and Retirement Study. Gerontologist 2017;57(2):319-28.
533. Arthur PB, Gitlin LN, Kairalla JA, Mann WC. Relationship
between the number of behavioral symptoms in dementia and
caregiver distress: What is the tipping point? Int Psychogeriatr
2018;30(8):1099-1107.
534. Solimando L, Fasulo M, Cavallero S, Veronese N, Smith L,
Vernuccio L. Suicide risk in caregivers of people with dementia:
a systematic review and meta-analysis. Aging Clin Exp Res
2022;34(10):2255-60.
535. Ivey-Stephenson AZ, Crosby AE, Hoenig JM, Gyawali S,
Park-Lee E, Hedden SL. Suicidal Thoughts and Behaviors
Among Adults Aged ≥18 Years — United States, 2015–2019.
MMWR Surveill Summ 2022;71(No. SS-1):1-19.
536. Gillespie R, Mullan J, Harrison L. Managing medications: The
role of informal caregivers of older adults and people living with
dementia: A review of the literature. J Clin Nurs 2014;23(23-
24):3296-308.
537. Alsaeed D, Jamieson E, Gul MO, Smith FJ. Challenges to
optimal medicines use in people living with dementia and their
caregivers: A literature review. Int J Pharm 2016;512(2):396-404.
538. Polenick CA, Stanz SD, Leggett AN, Maust DT, Hodgson NA,
Kales HC. Stressors and resources related to medication
management: Associations with spousal caregivers' role
overload. Gerontologist 2020;60(1):165-73
539. Aston L, Hilton A, Moutela T, Shaw R, Maidment I. Exploring the
evidence base for how people with dementia and their informal
carers manage their medication in the community: A mixed
studies review. BMC Geriatr 2017;17(1):242.
540. Liu C, Fabius CD, Howard VJ, Haley WE, Roth DL. Change in
Social Engagement among Incident Caregivers and Controls:
Findings from the Caregiving Transitions Study. J Aging Health
2021;33(1-2):114-24.
541. Anderson JG, Jabson Tree JM, Flatt JD, Gross AL, Williams IC,
Rose KM. A comparative analysis of family quality of life
between heterosexual and sexual minority caregivers of people
with dementia. J Appl Gerontol 2022;41(6):1576-84.
542. Lee J, Baik S, Becker TD, Cheon JH. Themes describing social
isolation in family caregivers of people living with dementia: A
scoping review. Dementia (London) 2022;21(2):701-21.
543. Alhasan DM, Hirsch JA, Jackson CL, Miller MC, Cai B, Lohman
MC. Neighborhood Characteristics and the Mental Health of
Caregivers Cohabiting with Care Recipients Diagnosed with
Alzheimer's Disease. Int J Environ Res Public Health
2021;18(3):913.
544. Vetter M, Donelan K, Guzikowski S, Michael C, Ritchie CS,
Vogeli C, et al. The needs of family caregivers of persons living
with dementia cared for in primary care practices. J Eval Clin
Pract 2023;29(8):1243-6.
545. Burgdorf JG, Amjad H. Impact of diagnosed (vs undiagnosed)
dementia on family caregiving experiences. J Am Geriatr Soc.
2023;71(4):1236-42.
546. Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Clinically
significant changes in burden and depression among dementia
caregivers following nursing home admission. BMC Medicine
2010;8:85.
547. Mausbach BT, Chattillion EA, Ho J, Flynn LM, Tiznado D, von
Känel R, et al. Why does placement of persons with Alzheimer’s
disease into long-term care improve caregivers’ well-being?
Examination of psychological mediators. Psychol Aging
2014;29(4):776-86.
548. Lee K, Chung J, Meyer KN, Dionne-Odom JN. Unmet needs
and health-related quality of life of dementia family caregivers
transitioning from home to long-term care: A scoping review.
Geriatr Nurs 2022;43:254-64.
549. Peacock SC. The experience of providing end-of-life care to a
relative with advanced dementia: An integrative literature
review. Palliat Support Care 2013;11(2):155-68.
550. Schulz R, Mendelsohn AB, Haley WE, Mahoney D, Allen RS,
Zhang S, et al. End-of-life care and the effects of bereavement
on family caregivers of persons with dementia. N Engl J Med
2003;349(20):1936-42.
551. Kumar V, Ankuda CK, Aldridge MD, Husain M, Ornstein KA.
Family Caregiving at the End of Life and Hospice Use: A
national study of Medicare beneficiaries. J Am Geriatr Soc
2020;68(10):2288-96.
552. Fonareva I, Oken BS. Physiological and functional
consequences of caregiving for relatives with dementia. Int
Psychogeriatr 2014;26(5):725-47.
553. Parker LJ, Fabius C, Rivers E, Taylor JL. Is Dementia-Specific
Caregiving Compared With Non-Dementia Caregiving
Associated With Physical Difficulty Among Caregivers for
Community-Dwelling Adults? J Appl Gerontol
2022;41(4):1074-80.
554. Peng H-L, Chang Y-P. Sleep disturbance in family caregivers of
individuals with dementia: A review of the literature. Perspect
Psychiatr C 2012;49(2):135-46.
555. Gao C, Chapagain NY, Scullin MK. Sleep Duration and Sleep
Quality in caregivers of patients with dementia: A systematic
review and meta-analysis. JAMA Netw Open 2019;2(8):e199891.
556. Liu Y, Song Y, Johnson FU, Lei L, Emily Choi S-W, Antonucci
TC, et al. Characteristics and predictors of sleep among spousal
care dyads living with chronic conditions. J Gerontol B Psychol
Sci Soc Sci 2023;78(Suppl 1):S38-S47.
557. Brewster GS, Wang D, McPhillips MV, Epps F, Yang I. Correlates
of Sleep Disturbance Experienced by Informal Caregivers of
Persons Living with Dementia: A Systematic Review. Clin
Gerontol 2022;1-28.
558. Brodaty H, Donkin M. Family caregivers of people with
dementia. Dialogues Clin Neurosci 2009;11(2):217-28.
559. von Känel R, Mausbach BT, Dimsdale JE, Ziegler MG, Mills PJ,
Allison MA, et al. Refining caregiver vulnerability for clinical
practice: Determinants of self-rated health in spousal dementia
caregivers. BMC Geriatr 2019;19(1):18.
560. Dassel KB, Carr DC. Does dementia caregiving accelerate
frailty? Findings from the Health and Retirement Study.
Gerontologist 2016;56(3):444-50.
561. Fredman L, Bertrand RM, Martire LM, Hochberg M, Harris EL.
Leisure-time exercise and overall physical activity in older
women caregivers and non-caregivers from the Caregiver-SOF
Study. Prev Med 2006;43:226-9.
562. Secinti E, Wu W, Kent EE, Demark-Wahnefried W, Lewson AB,
Mosher CE. Examining Health Behaviors of Chronic Disease
Caregivers in the U.S. Am J Prev Med 2022;62(3):e145-e158.
563. Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive
health effects of caring for a disabled spouse: Longitudinal
findings from the Caregiver Health Effects Study. Psychol
Aging 2000;15(2):259-71.
564. von Kanel R, Mausbach BT, Dimsdale JE, Mills PJ, Patterson TL,
Ancoli-Israel S, et al. Effect of chronic dementia caregiving and
major transitions in the caregiving situation on kidney function:
A longitudinal study. Psychosom Med 2012;74(2):214-20.
565. Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R.
Spousal caregivers of dementia victims: Longitudinal changes in
immunity and health. Psychosom Med 1991;53:345-62.
566. Kiecolt-Glaser JK, Marucha PT, Mercado AM, Malarkey WB,
Glaser R. Slowing of wound healing by psychological stress.
Lancet 1995;346(8984):1194-6.
567. Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB,
Siegler I. A path model of chronic stress, the metabolic
syndrome, and coronary heart disease. Psychosom Med
2002;64:418-35.
568. Mausbach BT, Romero-Moreno R, Bos T, von Känel R, Ziegler
MG, Allison MA, et al. Engagement in pleasant leisure activities
and blood pressure: A 5-year longitudinal study in Alzheimer
caregivers. Psychosom Med. 2017;79(7):735-41.
569. Shaw WS, Patterson TL, Ziegler MG, Dimsdale JE, Semple SJ,
Grant I. Accelerated risk of hypertensive blood pressure
recordings among Alzheimer caregivers. J Psychosom Res
1999;46(3):215-27.
570. Mausbach BT, Roepke SK, Ziegler MG, Milic M, Von Kanel R,
Dimsdale JE, et al. Association between chronic caregiving
stress and impaired endothelial function in the elderly. J Am
Coll Cardiol 2010;55(23):2599-606.
133
Appendices
571. Allen AP, Curran EA, Duggan Á, Cryan JF, Chorcoráin AN,
Dinan TG, et al. A systematic review of the psychobiological
burden of informal caregiving for patients with dementia: Focus
on cognitive and biological markers of chronic stress. Neurosci
Biobehav Rev 2017;73:123-64.
572. Roth DL, Sheehan OC, Haley WE, Jenny NS, Cushman M,
Walston JD. Is family caregiving associated with inflammation
or compromised immunity? A meta-analysis. Gerontologist
2019;59(5):e521-e534.
573. Roth DL, Haley WE, Sheehan OC, Huang J, Rhodes JD, Durda
P, et al. The transition to family caregiving and its effect on
biomarkers of inflammation. Proc Natl Acad Sci USA
2020;117(28):16258-63.
574. Schubert CC, Boustani M, Callahan CM, Perkins AJ, Hui S,
Hendrie HC. Acute care utilization by dementia caregivers
within urban primary care practices. J Gen Intern Med
2008;23(11):1736-40.
575. Zhu CW, Scarmeas N, Ornstein K, Albert M, Brandt J, Blacker
D, et al. Health-care use and cost in dementia caregivers:
Longitudinal results from the Predictors Caregiver Study.
Alzheimers Dement 2015;11(4):444-54.
576. Meyer K, Gassoumis Z, Wilber K. The Differential Effects of
Caregiving Intensity on Overnight Hospitalization. West J Nurs
Res 2022;44(6):528-39.
577. Leggett AN, Sonnega AJ, Lohman MC. Till death do us part:
Intersecting health and spousal dementia caregiving on
caregiver mortality. J Aging Health 2020;32(7-8):871-9.
578. Roth DL, Fredman L, Haley WE. Informal caregiving and its
impact on health: A reappraisal from population-based studies.
Gerontologist 2015;55(2):309-19.
579. Christakis NA, Allison PD. Mortality after the hospitalization of
a spouse. N Engl J Med 2006;354:719-30.
580. Perkins M, Howard VJ, Wadley VG, Crowe M, Safford MM,
Haley WE, et al. Caregiving strain and all-cause mortality:
Evidence from the REGARDS Study. J Gerontol B Psychol Sci
Soc Sci 2013;68(4):504-12.
581. Gaugler JE, Jutkowitz E, Peterson CM, Zmora R. Caregivers
dying before care recipients with dementia. Alzheimers Dement
(NY) 2018;4:688-93.
582. Kelley AS, McGarry K, Bollens-Lund E, Rahman O-K, Husain M,
Ferreira KB, et al. Residential setting and the cumulative
financial burden of dementia in the 7 years before death. J Am
Geriatr Soc 2020;68(6):1319-24.
583. AARP, Family Caregiving and Out-of-Pocket Costs: 2016
Report. Available at: https://www.aarp.org/content/dam/aarp/
research/surveys_statistics/ltc/2016/family-caregiving-costs-
fact-sheet.doi.10.26419%252Fres.00138.002.pdf. Accessed
December 15, 2023.
584. Albert SM. Are Medical Care Expenses Higher for Spouses
Who Provide Dementia Care? Am J Geriatr Psychiatry
2021;29(5):476-7.
585. Chu J, Benjenk I, Chen J. Incremental Health Care
Expenditures of the Spouses of Older Adults With Alzheimer's
Diseases and Related Dementias (ADRD). Am J Geriatr
Psychiatry 2021;29(5):462-72.
586. Stall NM, Kim SJ, Hardacre KA, Shah PS, Straus SE, Bronskill
SE, et al. Association of informal caregiver distress with health
outcomes of community-dwelling dementia care recipients: A
systematic review. J Am Geriatr So 2019;67(3):609-17.
587. Amjad H, Mulcahy J, Kasper JD, Burgdorf J, Roth DL, Covinsky
K, et al. Do caregiving factors affect hospitalization risk among
disabled older adults? J Am Geriatr Soc 2021;69(1):129-39.
588. Sullivan SS, de Rosa C, Li CS, Chang YP. Dementia caregiver
burdens predict overnight hospitalization and hospice
utilization. Palliat Support Care 2022 Oct 20;1-15.
589. Hennelly N, Cooney A, Houghton C, O'Shea E. Personhood and
Dementia Care: A Qualitative Evidence Synthesis of the
Perspectives of People With Dementia. Gerontologist
2021;61(3):e85-e100.
590. Cheng S-K, Li K-K, Or PPL, Losada A. Do caregiver
interventions improve outcomes in relatives with dementia and
mild cognitive impairment? A comprehensive systematic review
and meta-analysis. Psychol Aging 2022;37(8):929-53.
591. Gaugler JE, Jutkowitz E, Shippee TP, Brasure M. Consistency
of dementia caregiver intervention classification: An evidence-
based synthesis. Int Psychogeriatr 2017;29(1):19-30.
592. Gitlin LN, Hodgson N. Caregivers as therapeutic agents in
dementia care: The evidence-base for interventions supporting
their role. In: Gaugler JE, Kane RL, eds. Family caregiving in the
new normal. Philadelphia, Pa.: Elsevier, Inc.; 2015: p. 305-56.
593. Liew TM, Lee CS. Reappraising the efficacy and acceptability of
multicomponent interventions for caregiver depression in
dementia: The utility of network meta-analysis. Gerontologist
2019;16;59(4):e380-e392.
594. Williams F, Moghaddam N, Ramsden S, De Boos D.
Interventions for reducing levels of burden amongst informal
carers of persons with dementia in the community. A
systematic review and meta-analysis of randomised controlled
trials. Aging Ment Health 2019;23(12):1629-42.
595. Kaddour L, Kishita N, Schaller A. A meta-analysis of low-
intensity cognitive behavioral therapy-based interventions for
dementia caregivers. Int Psychogeriatr 2018:1-16.
596. Nguyen H, Terry D, Phan H, Vickers J, McInerney F.
Communication training and its effects on carer and care-
receiver outcomes in dementia settings: A systematic review. J
Clin Nurs 2019;28(7-8):1050-69.
597. Jütten LH, Mark RE, Wicherts JM, Sitskoorn MM. The
effectiveness of psychosocial and behavioral interventions for
informal dementia caregivers: Meta-analyses and meta-
regressions. J Alzheimers Dis 2018;66(1):149-72.
598. Maslow K. Translating Innovation to Impact: Evidence-Based
Interventions to Support People with Alzheimer’s Disease
and their Caregiver at Home and in the Community.
Washington, D.C.: Administration on Aging; 2012. Available at:
https://www.agingresearch.org/app/uploads/2017/12/50820C
ompliant20AoA-White-Paper20for20Release.pdf. Accessed
December 15, 2023.
599. Rosalynn Carter Institute for Caregiving. Available at: https://
www.rosalynncarter.org/. Accessed December 15, 2023.
600. Larson EB, Stroud C. Meeting the Challenge of Caring for
Persons Living With Dementia and Their Care Partners and
Caregivers: A Report From the National Academies of Sciences,
Engineering, and Medicine. JAMA 2021;325(18):1831-2.
601. Cheng ST, Li KK, Or PPL, Losada A. Do caregiver interventions
improve outcomes in relatives with dementia and mild cognitive
impairment? A comprehensive systematic review and
meta-analysis. Psychol Aging 2022;37(8):929-53.
602. Cheng S-T, Li K-K, Losada A, Zhang F, Au A, Thompson LW,
et al. The effectiveness of nonpharmacological interventions
for informal dementia caregivers: An updated systematic review
and meta-analysis. Psychol Aging 2020;35(1):55-77.
603. Walter E, Pinquart M. How Effective Are Dementia Caregiver
Interventions? An Updated Comprehensive Meta-Analysis.
Gerontologist 2020;60(8):609-19.
604. Gitlin LN, Jutkowitz E, Gaugler JE. Dementia caregiver
intervention research now and into the future: Review and
recommendations. Washington, D.C.: Commissioned paper for
the National Academies of Science, Engineering and Medicine
NIA Decadal Study. Available at: https://sites.nationalacademies.
org/cs/groups/dbassesite/documents/webpage/
dbasse_198208.pdf. Accessed December 15, 2023.
605. Lee M, Ryoo JH, Chung M, Anderson JG, Rose K, Williams IC.
Effective interventions for depressive symptoms among
caregivers of people with dementia: A systematic review and
meta-analysis. Dementia (London) 2020;19(7):2368-98.
606. Cheng S-T, Zhang F. A comprehensive meta-review of
systematic reviews and meta-analyses on nonpharmacological
interventions for informal dementia caregivers. BMC Geriatr
2020;20(1):137.
607. Perales-Puchalt J, Barton K, Ptomey L, Niedens M, Yeager A,
Gilman L, et al. Effectiveness of "Reducing Disability in
Alzheimer's Disease" Among Dyads With Moderate Dementia. J
Appl Gerontol 2021;40(10):1163-71.
608. Bass DM, Hornick T, Kunik M, Judge KS, Primetica B, Kearney
K, et al. Findings from a real-world translation study of the
evidence-based "Partners in Dementia Care". Innov Aging
2019;3(3):igz031.
134 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
609. Hodgson N, Gitlin LN (in press). Implementing and sustaining
family care programs in real world settings: Barriers and
facilitators. In J. E. Gaugler (Ed.), Bridging the Family Care Gap.
Academic Press: San Diego, CA.
610. Fauth EB, Jackson MA, Walberg DK, Lee NE, Easom LR, Alston
G, et al. External validity of the New York University Caregiver
Intervention: Key caregiver outcomes across multiple
demonstration projects. J Appl Gerontol 2019;38(9):1253-81.
611. Hodgson NA, Petrovsky DV, Finegan K, Kallmyer BA, Pike J,
Fazio S. One call makes a difference: An evaluation of the
Alzheimer's Association National Helpline on dementia
caregiver outcomes. Patient Educ Couns 2021;104(4):896-902.
612. Reuben DB, Evertson LC, Jackson-Stoeckle R, Epstein-Lubow
G, Spragens LH, Haggerty KL. Dissemination of a successful
dementia care program: Lessons to facilitate spread of
innovations. J Am Geriatr Soc 2022;70(9):2686-94.
613. Gitlin LN, Roth DL, Marx K, Parker LJ, Koeuth S, Dabelko-
Schoeny H, et al. Embedding caregiver support within adult day
services: Outcomes of a multi-site trial. Gerontologist 2023
Aug 7:gnad107.
614. Boustani M, Alder CA, Solid CA. Agile implementation: A
blueprint for implementing evidence-based healthcare
solutions. J Am Geriatr Soc 2018;66(7):1372-6.
615. Boots LM, de Vugt ME, van Knippenberg RJ, Kempen GI,
Verhey FR. A systematic review of internet-based supportive
interventions for caregivers of patients with dementia. Int J
Geriatr Psych 2015;29(4):331-44.
616. Griffiths PC, Whitney MK, Kovaleva M, Hepburn K.
Development and implementation of tele-savvy for dementia
caregivers: A Department of Veterans Affairs Clinical
Demonstration Project. Gerontologist 2016;56(1):145-54.
617. Gaugler JE, Zmora R, Mitchell LL, Finlay JM, Peterson CM,
McCarron H, et al. Six-month effectiveness of remote activity
monitoring for persons living with dementia and their family
caregivers: An experimental mixed methods study.
Gerontologist 2019;9;59(1):78-89
618. Waller A, Dilworth S, Mansfield E, Sanson-Fisher R. Computer
and telephone delivered interventions to support caregivers of
people with dementia: A systematic review of research output
and quality. BMC Geriatr 2017;17(1):265.
619. Hopwood J, Walker N, McDonagh L, Rait G, Walters K, Iliffe S,
et al. Internet-based interventions aimed at supporting family
caregivers of people with dementia: Systematic review. J Med
Internet Res 2018;12;20(6):e216.
620. Leng M, Zhao Y, Xiao H, Li C, Wang Z. Internet-based
supportive interventions for family caregivers of people with
dementia: Systematic review and meta-analysis. J Med Internet
Res 2020;22(9):e19468.
621. Pleasant M, Molinari V, Dobbs C, Meng H, Hyer K. Effectiveness
of online dementia caregivers training programs: A systematic
review. Geriatr Nurs 2020;S0197-4572(20):30209-3.
622. Etxeberria I, Salaberria K, Gorostiaga A. Online support for
family caregivers of people with dementia: a systematic review
and meta-analysis of RCTs and quasi-experimental studies.
Aging Ment Health 2021;25(7):1165-80.
623. Saragih ID, Tonapa SI, Porta CM, Lee BO. Effects of telehealth
intervention for people with dementia and their carers: A
systematic review and meta-analysis of randomized controlled
studies. J Nurs Schol 2022;54(6):704-19.
624. Fortinsky RH, Gitlin LN, Pizzi LT, Piersol CV, Grady J, Robison
JT, et al. Effectiveness of the care of persons with dementia in
their environments intervention when embedded in a publicly
funded home- and community-based service program. Innov
Aging 2020;4(6):igaa053.
625. Haggerty KL, Campetti R, Stoeckle RJ, Epstein-Lubow G,
Evertson LC, Spragens L. Dissemination of a successful
dementia care program: Lessons from early adopters. J Am
Geriatr Soc 2022;70(9):2677-85.
626. Maslow K, Bass DM, Rentsch JH. Update on the status of
effective programs to help dementia family caregivers in the
United States: Observations from the search for programs to
include in Best Practice Caregiving. In Gaugler JE, ed. Bridging
the Family Care Gap. Academic Press; 2021:247-302.
627. Gaugler JE, Potter T, Pruinelli L. Partnering with caregivers.
Clin Geriatr Med 2014;30(3):493-515.
628. Gitlin LN, Marx K, Stanley IH, Hodgson N. Translating evidence-
based dementia caregiving interventions into practice:
State-of-the-science and next steps. Gerontologist
2015;55(2):210-26.
629. Wethington E, Burgio LD. Translational research on caregiving:
Missing links in the translation process. In: Gaugler JE, Kane RL,
eds. Family caregiving in the new normal. Philadelphia, Pa.:
Elsevier, Inc; 2015: p. 193-210.
630. Zarit SH. Past is prologue: How to advance caregiver
interventions. Aging Ment Health 2017;16:1-6.
631. Gonella S, Mitchell G, Bavelaar L, Conti A, Vanalli M, Basso I.
Interventions to support family caregivers of people with
advanced dementia at the end of life in nursing homes: A
mixed-methods systematic review. Palliat Med 2022;36(2):268-91.
632. Kishita N, Hammond L, Dietrich CM, Mioshi E. Which
interventions work for dementia family carers?: an updated
systematic review of randomized controlled trials of carer
interventions. Int Psychogeriatr 2018;30(11):1679-96.
633. Zarit SH, Lee JE, Barrineau MJ, Whitlatch CJ, Femia EE.
Fidelity and acceptability of an adaptive intervention for
caregivers: An exploratory study. Aging Ment Health
2013;17(2):197-206.
634. Van Mierlo LD, Meiland FJ, Van Hout HP, Dröes RM. Toward an
evidence-based implementation model and checklist for
personalized dementia care in the community. Int
Psychogeriatr 2016;28(5):801-13.
635. Gaugler JE, Reese M, Tanler R. Care to Plan: An online tool that
offers tailored support to dementia caregivers. Gerontologist
2016;56(6):1161-74.
636. Jennings LA, Ramirez KD, Hays RD, Wenger NS, Reuben DB.
Personalized goal attainment in dementia care: Measuring what
persons with dementia and their caregivers want. J Am Geriatr
Soc 2018;66(11):2120-7.
637. Whitlatch CJ, Orsulic-Jeras S. Meeting the informational,
educational, and psychosocial support needs of persons living
with dementia and their family caregivers. Gerontologist
2018;18;58(suppl_1):S58-73.
638. Akarsu NE, Prince MJ, Lawrence VC, Das-Munshi J. Depression
in carers of people with dementia from a minority ethnic
background: Systematic review and meta-analysis of
randomised controlled trials of psychosocial interventions. Int J
Geriatr Psychiatry 2019;34(6):790-806.
639. Llanque SM, Enriquez M. Interventions for Hispanic caregivers
of patients with dementia: A review of the literature. Am J
Alzheimers Dis Other Demen 2012;27(1):23-32.
640. Napoles AM, Chadiha L, Eversley R, Moreno-John G. Reviews:
Developing culturally sensitive dementia caregiver
interventions: Are we there yet? Am J Alzheimers Dis Other
Dement 2010;25:389-406.
641. Luchsinger JA, Burgio L, Mittelman M, Dunner I, Levine JA,
Hoyos C, et al. Comparative effectiveness of 2 interventions
for Hispanic caregivers of persons with dementia. J Am Geriatr
Soc 2018;66(9):1708-15.
642. Fredriksen-Goldsen KI, Jen S, Bryan AEB, Goldsen J. Cognitive
impairment, Alzheimer's disease, and other dementias in the
lives of lesbian, gay, bisexual and transgender (LGBT) older
adults and their caregivers: Needs and competencies. J Appl
Gerontol 2018;37(5):545-69.
643. U.S. Department of Health and Human Services. National
Research Summit on Care, Services and Supports for Persons
with Dementia and their Caregivers. Available at: https://aspe.
hhs.gov/national-research-summit-care-services-and-
supports-persons-dementia-and-their-caregivers. Accessed
December 15, 2023.
644. Young HM, Bell JF, Whitney RL, Ridberg RA, Reed SC, Vitaliano
PP. Social determinants of health: Underreported
heterogeneity in systematic reviews of caregiver interventions.
Gerontologist 2020;60(Suppl 1):S14-S28.
135
Appendices
645. Brewster GS, Epps F, Dye CE, Hepburn K, Higgins MK, Parker
ML. The effect of the "Great Village" on psychological
outcomes, burden, and mastery in African American caregivers
of persons living with dementia. J Appl Gerontol
2020;39(10):1059-68.
646. Demanes A, Ward KT, Wang AT, Hess M. Systematic Review of
Dementia Support Programs with Multicultural and Multilingual
Populations. Geriatrics (Basel) 2021;7(1):8.
647. Di Lorito C, Bosco A, Peel E, Hinchliff S, Dening T, Calasanti T.
Are dementia services and support organisations meeting the
needs of Lesbian, Gay, Bisexual and Transgender (LGBT)
caregivers of LGBT people living with dementia? A scoping review
of the literature. Aging Ment Health 2022;26(10):1912-21.
648. Kittle KR, Lee R, Pollock K, Song Y, Wharton W, Andersonet
JG, et al. Feasibility of the Savvy Caregiver Program for
LGBTQ+ Caregivers of people living with Alzheimer's disease
and related dementias. Int J Environ Res Pub Health
2022;19(22):15102.
649. Alzheimer's Association. Alzheimer's Association Dementia
Care Practice Recommendations. Available at: https://www.alz.
org/media/Documents/alzheimers-dementia-care-practice-
recommendations.pdf. Accessed December 15, 2023.
650. Camp CJ. Denial of human rights: We must change the
paradigm of dementia care. Clin Gerontol 2019;42(3):221-3.
651. Gaugler JE, Bain LJ, Mitchell L, Finlay J, Fazio S, Jutkowitz E,
et al. Reconsidering frameworks of Alzheimer's dementia when
assessing psychosocial outcomes. Alzheimers Dement (NY)
2019;5:388-97.
652. Burton A, Ogden M, Cooper C. Planning and enabling
meaningful patient and public involvement in dementia
research. Curr Opin Psychiatry 2019;32(6):557-62.
653. The Lewin Group. Process Evaluation of the Older Americans
Act Title IIIE-National Family Caregiver Support Program: Final
Report, 2016. Available at: https://acl.gov/sites/default/files/
programs/2017-02/NFCSP_Final_Report-update.pdf.
Accessed December 15, 2023.
654. Stone RI. Factors affecting the future of family caregiving in
the United States. In: JE Gaugler, RL Kane, eds. Family
Caregiving in the New Normal. San Diego, CA: Elsevier, Inc;
2015: p. 57-77.
655. Gaugler JE. Supporting family care for older adults: Building a
better bridge. In J. E. Gaugler (Ed.). Bridging the Family Care
Gap. Academic Press.; 2021: p. 427-52.
656. Gaugler JE, Borson S, Epps F, Shih RA, Parker LJ, McGuire LC.
The intersection of social determinants of health and family
care of people living with Alzheimer's disease and related
dementias: A public health opportunity. Alzheimers Dement
2023;10.1002/alz.13437.
657. Greenberg NE, Wallick A, Brown LM. Impact of COVID-19
pandemic restrictions on community-dwelling caregivers and
persons with dementia. Psychol Trauma
2020;12(S1):S220-S221.
658. Carbone EA, de Filippis R, Roberti R, Rania M, Destefano L,
Russo E. The Mental Health of Caregivers and Their Patients
With Dementia During the COVID-19 Pandemic: A Systematic
Review. Front Psychol 2021;12:782833.
659. Gaigher JM, Lacerda IB, Dourado MCN. Dementia and Mental
Health During the COVID-19 Pandemic: A Systematic Review.
Front Psychiatry 2022;13:879598.
660. Oliver S, Alexander K, Bennett SG, Hepburn K, Henry J,
Clevenger CK. Experiences of Black American Dementia
Caregivers During the COVID-19 Pandemic. J Fam Nurs
2022;28(3):195-204.
661. Perales-Puchalt J, Peltzer J, Fracachan-Cabrera M, Perez A,
Ramirez-Mantilla M, Greiner KA. Impact of the COVID-19
pandemic on Latino families with Alzheimer's disease and
related dementias: Perceptions of family caregivers and primary
care providers. medRxiv 2022;2022.05.25.22275517.
662. Masoud S, Glassner AA, Mendoza M, Rhodes S, White CL.
"A Different Way to Survive": The Experiences of Family
Caregivers of Persons Living With Dementia During the
COVID-19 Pandemic. J Fam Nurs 2022;28(3):243-57.
663. Turner RL, Reese-Melancon C, Harrington EE, Andreo M.
Caregiving during the COVID-19 pandemic: Factors associated
with feelings of caregiver preparedness. J Appl Gerontol
2023;42(10):2089-99.
664. Humber MB, Yefimova M, Lessios AS, Trivedi RB, Sheffrin M,
Martin M. "It isn't the same": Experiences of informal
caregivers of older adults enrolled in a home-based senior
care program during COVID-19. J Gerontol Nurs
2023;49(3):19-26.
665. Shrestha P, Fick DM, Boltz M, Loeb SJ, High AC. Caregiving for
persons living with dementia during the COVID-19 pandemic:
Perspectives of family care partners. J Gerontol Nurs
2023;49(3):27-33.
666. Arthur P, Li CY, Southern Indiana Dementia Workgroup. Living
with dementia during the COVID-19 pandemic: A nationwide
survey informing the American experience. J Alzheimers Dis
Rep 2022;6(1):733-7.
667. Chyu J, Cantu P, Mehta N, Markides K. Caregiving for people
with dementia or cognitive impairment during the COVID-19
pandemic: A review. Gerontol Geriatr Med
2022;8:23337214221132369.
668. Friedman EM, Kirkegaard A, Kennedy DP, Edgington S, Shih
RA. Change in Caregiving to Older Adults During the
COVID-19 Pandemic: Differences by Dementia Status. J Appl
Gerontol 2023;Sep 8:7334648231197514.
669. Macchi ZA, Ayele R, Dini M, Lamira J, Katz M, Pantilat SZ, et al.
Lessons from the COVID-19 pandemic for improving
outpatient neuropalliative care: A qualitative study of patient
and caregiver perspectives. Palliat Med 2021;35(7):1258-66.
670. Hwang Y, Connell LM, Rajpara AR, Hodgson NA. Impact of
COVID-19 on Dementia Caregivers and Factors Associated
With their Anxiety Symptoms. Am J Alzheimers Dis Other
Demen 2021;36:15333175211008768.
671. Savla J, Roberto KA, Blieszner R, McCann BR, Hoyt E, Knight
AL. Dementia caregiving during the "stay-at-home" phase of
COVID-19 pandemic. J Gerontol B Psychol Sci Soc Sci
2021;76(4):e241-e245.
672. Kusmaul N, Miller VJ, Cheon JH. "They Just Took Him Out of
My Life": Nursing Home Care Partner Experiences During the
COVID-19 Pandemic. J Gerontol Nurs 2022;48(2):7-11.
673. Mitchell LL, Albers EA, Birkeland RW, Peterson CM, Stabler H,
Horn B, et al. Caring for a relative with dementia in long-term
care during COVID-19. J Am Med Dir Assoc 2022;23(3):428-
33.e1.
674. Brungardt A, Cassidy J, LaRoche A, Dulaney S, Sawyer RJ,
Possin KL, et al. End-of-Life Experiences Within a Dementia
Support Program During COVID-19: Context and
Circumstances Surrounding Death During the Pandemic. Am J
Hosp Palliat Care 2022;10499091221116140.
675. Hunt JFV, Le Caire TJ, Schroeder M, O'Toole Smith K,
Marschall K, Walaszek A. The use of health care and
community-based services by people living with dementia and
their caregivers during the COVID-19 pandemic. WMJ
2022;121(3):226-30.
676. Gaugler JE. Our vast family care system for the elderly is at
risk of collapse. Available at: https://www.startribune.com/
our-vast-family-care-system-for-the-elderly-is-about-to-
collapse/572221182/. Accessed December 15, 2023.
677. Sadarangani T, Zhong J, Vora P, Missaelides L. "Advocating
Every Single Day" so as Not to be Forgotten: Factors
Supporting Resiliency in Adult Day Service Centers Amidst
COVID-19-Related Closures. J Gerontol Soc Work
2021;64(3):291-302.
678. Gaugler JE, Marx K, Dabelko-Schoeny H, Parker L, Anderson
KA, Albers E, et al. COVID-19 and the Need for Adult Day
Services. J Am Med Dir Assoc 2021;22(7):1333-7.
679. Monin JK, Ali T, Syed S, Piechota A, Lepore M, Mourgues C,
et al. Family communication in long-term care during a
pandemic: Lessons for enhancing emotional experiences. Am J
Geriatr Psychiatry 2020;S1064-7481(20):30478-4.
136 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
680. Pickering CEZ, Maxwell CD, Yefimova M, Wang D, Puga F,
Sullivan T. Early Stages of COVID-19 Pandemic Had No
Discernable Impact on Risk of Elder Abuse and Neglect Among
Dementia Family Caregivers: A Daily Diary Study. J Fam
Violence 2022;5:1-11.
681. Albers EA, Mikal J, Millenbah A, Finlay J, Jutkowitz E, Mitchell
L. The Use of Technology Among Persons With Memory
Concerns and Their Caregivers in the United States During the
COVID-19 Pandemic: Qualitative Study. JMIR Aging
2022;5(1):e31552.
682. Yoon SO, Paek EJ. Video call usage in older adults with or
without dementia impacted by the COVID-19 pandemic. Am J
Alzheimers Dis Other Demen 2023;38:15333175231160679.
683. O'Connor MK, Nicholson R, Epstein C, Donley T, Salant R,
Nguyen AH, et al. Telehealth support for dementia caregivers
during the COVID-19 pandemic: Lessons learned from the
NYU family support program. Am J Geriatr Psychiatry
2023;31(1):14-21.
684. Yan K, Sadler T, Brauner D, Pollack HA, Konetzka RT. Caregiving
for older adults with dementia during the time of COVID-19: A
multi-state exploratory qualitative study. J Appl Gerontol
2023;42(10):2078-88.
685. Nkodo J-A, Gana W, Debacq C, Aidoud A, Poupin P, Camus V,
et al. The Role of Telemedicine in the Management of the
Behavioral and Psychological Symptoms of Dementia: A
Systematic Review. Am J Geriatr Psychiatry 2022;30(10):
1135-50.
686. Liang J, Aranda MP. The use of telehealth among people living
with dementia-caregiver dyads during the COVID-19
pandemic: Scoping review. J Med Internet Res
2023;25:e45045.
687. Lloyd SL, Caban-Holt A, Starks TD, Clark JC, Byrd GS.
Assessing gender differences on the impact of COVID-19 on
the medical and social needs of dementia caregivers. J
Gerontol Soc Work 2023 Aug 14;1-16.
688. National Institute on Aging. NIA COVID-19 Response. Available
at: https://www.nia.nih.gov/research/grants-funding/
nia-covid-19-response. Accessed December 4, 2023.
689. Weems JA, Rhodes S, Powers JS. Dementia Caregiver Virtual
Support-An Implementation Evaluation of Two Pragmatic
Models during COVID-19. Geriatrics (Basel) 2021;6(3):80.
690. Masoud SS, Meyer KN, Sweet LM, Prado PJ, White CL. "We
Don't Feel so Alone": A Qualitative Study of Virtual Memory
Cafés to Support Social Connectedness Among Individuals
Living With Dementia and Care Partners During COVID-19.
Front Public Health 2021;9:660144.
691. Administration for Community Living. 2022 National Strategy
to Support Family Caregivers. Available at: https://acl.gov/sites/
default/files/RAISE_SGRG/
NatlStrategyToSupportFamilyCaregivers.pdf. Accessed
December 15, 2023.
692. U.S. Centers for Medicare & Medicaid Services. Guiding an
Improved Dementia Experience (GUIDE) Model. Available at:
https://www.cms.gov/priorities/innovation/innovation-models/
guide. Accessed December 4, 2023.
693. American Public Health Association. (2020)Strengthening the
dementia care workforce: A public health priority. Available at:
https://www.apha.org/policies-and-advocacy/public-health-
policy-statements/policy-database/2021/01/13/
strengthening-the-dementia-care-workforce.
694. Alzheimer’s Association and Centers for Disease Control and
Prevention. Healthy Brain Initiative, State and Local Public
Health Partnerships to Address Dementia: The 2018–2023
Road Map. Chicago, IL: Alzheimer’s Association; 2018. https://
www.cdc.gov/aging/pdf/2018-2023-Road-Map-508.pdf.
695. Schneider J , Jeon S , Gladman JT , Corriveau RA. ADRD
Summit 2019 Report to the National Advisory Neurological
Disorders and Stroke Council. https://aspe.hhs.gov/
collaborations-committees-advisory-groups/napa/napa-
documents/napa-research-milestones/adrd-summit-2019-
prioritized-research-milestones,.
696. U.S. Department of Health and Human Services. National Plan
to Address Alzheimer’s Disease: 2020 Update. https://aspe.hhs.
gov/reports/national-plan-address-alzheimers-disease-2020-
update-0.
697. Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and
Figures. Special report: more than normal aging: understanding
mild cognitive impairment. Available at: https://doi.
org/10.1002/alz.12638. Accessed December 15, 2023.
698. Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected
and diagnosed rates of mild cognitive impairment and dementia
in the U.S. Medicare population: observational analysis.
Alzheimers Res Ther 2023;15(1):128.
699. Owens DK , Davidson KW , Krist AH , Barry MJ , Cabana M ,
Caughey AB , et al. Screening for cognitive impairment in older
adults: US Preventive Services Task Force Recommendation
Statement. JAMA 2020;323: 757-63.
700. Petersen RC , Lopez O , Armstrong MJ , Getchius TSD , Ganguli
M , Gloss D , et al. Practice guideline update summary: Mild
cognitive impairment: Report of the Guideline Development,
Dissemination, and Implementation Subcommittee of the
American Academy of Neurology. Neurology 2018;90:126-35.
701. Cordell CB , Borson S , Boustani M , Chodosh J , Reuben D ,
Verghese J , et al. Alzheimer’s Association recommendations
for operationalizing the detection of cognitive impairment
during the Medicare Annual Wellness Visit in a primary care
setting. Alzheimers Dement 2013;9:141-50.
702. Galvin JE , Sadowsky CH. Practical guidelines for the
recognition and diagnosis of dementia. J Am Board Fam Med
2012;25:367-82.
703. 2020 Alzheimer's disease facts and figures. Alzheimers Dement
2020;doi:10.1002/alz.12068.
704. Bernstein A , Rogers KM , Possin KL , Steele NZR , Ritchie CS ,
Kramer JH , et al. Dementia assessment and management in
primary care settings: A survey of current provider practices in
the United States. BMC Health Serv Res 2019;19: 919.
705. Neprash HT, Everhart A, McAlpine D, Smith LB, Sheridan B,
Cross DA. Measuring primary care exam length using
electronic health record data. Med care 2021;59(1):62-6.
706. 706. Sabbagh MN, Boada M, Borson S, Chilukuri A, Dubois B,
Ingram J, et al. Early Detection of Mild Cognitive Impairment
(MCI) in Primary Care. J Prev Alzheimers Dis 2020;7(3):165–170.
707. Liss JL, Seleri Assunção S, Cummings J, Atri A, Geldmacher DS,
Candela SF, et al. Practical recommendations for timely,
accurate diagnosis of symptomatic Alzheimer's disease (MCI
and dementia) in primary care: a review and synthesis. J Intern
Med 2021;290(2):310-34.
708. Bernstein A, Rogers KM, Possin KL, Steele NZR, Ritchie CS,
Kramer JH et al. Dementia assessment and management in
primary care settings: a survey of current provider practices in
the United States. BMC Health Serv Res 2019;19: 919.
709. Drabo EF, Barthold D, Joyce G, Ferido P, Chui HC,
Zissimopoulos J. Longitudinal analysis of dementia diagnosis
and specialty care among racially diverse Medicare
beneficiaries. Alzheimers Dement 2019;15:1402-11.
710. Kotagal V , Langa KM , Plassman BL , Fisher GG , Giordani BJ ,
Wallace RB , et al. Factors associated with cognitive evaluations
in the United States. Neurology 2015;84:64-71.
711. Bradford A , Kunik ME , Schulz P , Williams SP , Singh H. Missed
and delayed diagnosis of dementia in primary care. Alzheimer
Dis Assoc Disord 2009;23:306-14.
712. Tsoy E , Kiekhofer RE , Guterman EL , Tee BL , Windon C ,
Dorsman KA , et al. Assessment of racial/ethnic disparities in
timeliness and comprehensiveness of dementia diagnosis in
California. JAMA Neurol 2021;6:657-65.
713. Lee SA, Kim D, Lee H. Examine Race/Ethnicity Disparities in
Perception, Intention, and Screening of Dementia in a
Community Setting: Scoping Review. Int J Environ Res Pub
Health 2022;19(14):8865.
714. Nguyen HQ, Borson S, Khang P, Langer-Gould A, Wanf SE,
Carrol J, et al. Dementia diagnosis and utilization patterns in a
racially diverse population within an integrated health care
delivery system. Alzheimers Dement (N Y) 2022;8(1):e12279.
137
Appendices
715. Davis MA, Lee KA, Harris M, Ha J, Langa KM, Bynum JPW, et al.
Time to dementia diagnosis by race: A retrospective cohort
study. JAGS 2022;70(11):3250-9.
716. U.S. Centers for Medicare & Medicaid Services. Cognitive
Assessment and Care Plan Services. Available at: https://www.
cms.gov/medicare/payment/fee-schedules/physician/
cognitive-assessment#:~:text=If%20your%20patient%20
shows%20signs,to%20bill%20for%20this%20service. Accessed
December 15, 2023.
717. Medicare.gov. Cognitive assessment & care plan services.
Available at: https://www.medicare.gov/coverage/cognitive-
assessment-care-plan-services. Accessed December 15, 2023.
718. Centers for Medicare & Medicaid Services. Billing and Coding:
Cognitive Assessment and Care Plan Service. Available at:
https://www.cms.gov/medicare-coverage-database/view/article.
aspx?articleid=59036&ver=11&. Accessed December 15, 2023.
719. Alzheimer's Association. Cognitive Assessment and Care
Planning Services: Alzheimer’s Association Expert Task Force
Recommendations and Tools for Implementation. Available at:
https://www.alz.org/careplanning/downloads/cms-consensus.
pdf. Accessed December 15, 2023.
720. Alzheimer's Association Fact Sheet: CPT® Code 99483,
Explanatory Guide for Clinicians. Available at: https://portal.
alzimpact.org/media/serve/id/5ab10bc1a3f3c. Accessed
December 15, 2023.
721. Hinton L , Franz CE , Reddy G , Flores Y , Kravitz RL , Barker JC.
Practice constraints, behavioral problems, and dementia care:
Primary care physicians’ perspectives. J Gen Intern Med
2007;22:1487-92.
722. Bernstein A , Rogers KM , Possin KL , Steele NZR , Ritchie CS ,
Miller BL , et al. Primary care provider attitudes and practices
evaluating and managing patients with neurocognitive
disorders. J Gen Intern Med 2019;34:1691-2.
723. U.S. Government Accountability Office. Medicare Cognitive
Assessments: Utilization Tripled between 2018 and 2022, but
Challenges Remain. December 11, 2023. Available at:https://
www.gao.gov/products/gao-24-106328. Accessed December
17, 2023.
724. Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and
Figures. Special report: on the front lines: primary care
physicians and Alzheimer's care in America. Available at: https://
alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.12068.
Accessed December 15, 2023.
725. American Geriatrics Society. (n.d.) State of the geriatric
workforce. Geriatrics workforce by the numbers. https://www.
americangeriatrics.org/geriatrics-profession/about-geriatrics/
geriatrics-workforce-numbers.
726. American Geriatrics Society. (2022) Current number of board
certified geriatricians by state. https://www.americangeriatrics.
org/sites/default/files/inline-files/Current%20Number%20
of%20Board%20Certified%20Geriatricians%20by%20State%20
%28July%202022%29.pdf.
727. IQVIA (a global provider of advanced analytics, technology
solutions, and clinical research services to the life sciences
industry) provided initial data for the number of geriatricians
(estimated at 5,170) under contract with the Alzheimer's
Association on December 1, 2021.
728. U.S. Department of Health and Human Services, Health
Resources and Services Administration, National Center for
Health Workforce Analysis. National and Regional Projections
of Supply and Demand for Geriatricians: 2013-2025. Available
at: https://bhw.hrsa.gov/sites/default/files/bureau-health-
workforce/data-research/geriatrics-report-51817.pdf.
Accessed December 15, 2023.
729. Health Resources & Services Administration. (n.d.) Workforce
projections. https://data.hrsa.gov/topics/health-workforce/
workforce-projections.
730. Andrilla CHA, Patterson DG, Garberson LA, Coulthard C,
Larson EH. Geographic variation in the supply of selected
behavioral health providers. Am J Prev Med 2018;54(6, Suppl
3):S199-S207.
731. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some
important barriers to health care access in the rural USA. Pub
health 2015;129(6):611-20.
732. National Resident Matching Program. Results and Data:
Specialties Matching Service. Washington, DC. 2023. Available
at: https://www.nrmp.org/wp-content/
uploads/2023/04/2023-SMS-Results-and-Data-Book.pdf.
Accessed December 17, 2023.
733. U.S. Department of Health and Human Services, Health
Resources and Services Administration, National Center for
Health Workforce Analysis. Health Workforce Projections:
Neurology Physicians and Physician Assistants. Available at:
https://bhw.hrsa.gov/sites/default/files/bureau-health-
workforce/data-research/bhw-factsheet-neurology.pdf.
Accessed December 15, 2023.
734. Rao A, Manteau-Rao M, Aggarwal NT. Dementia neurology
deserts: what are they and where are they located in the US?
Alzheimers Dement. 2017;13(suppl 7):P509.
735. Majersik JJ, Ahmed A, Chen IH, Shill H, Hanes GP, Pelak VS,
et al. A Shortage of Neurologists–We Must Act Now: A Report
From the AAN 2019 Transforming Leaders Program.
Neurology 2021;96(24):1122-34.
736. Warshaw GA, Bragg EJ. Preparing the health care workforce
to care for adults with Alzheimer’s disease and related
dementias. Health Aff 2014;33(4):633-41.
737. Yang M, Chang CH, Carmichael D, Oh ES, Bynum JP. Who is
providing the predominant care for older adults with dementia?
J Am Med Dir Assoc 2016;17(9):802-6.
738. Warshaw GA, Bragg EJ. The essential components of quality
geriatric care. Generations 2016;40(1):28-37.
739. Auerbach DI, Buerhaus PI, Staiger DO. Implications of the rapid
growth of the nurse practitioner workforce in the US: An
examination of recent changes in demographic, employment,
and earnings characteristics of nurse practitioners and the
implications of those changes. Health Aff 2020;39(2):273-9.
740. American Association of Nurse Practitioners (AANP). NP Fact
Sheet 2022. Available at: https://www.aanp.org/about/
all-about-nps/np-fact-sheet. Accessed December 15, 2023.
741. American Board of Psychiatry and Neurology. Certifications by
Year - Subspecialities. Available at: https://www.abpn.org/
wp-content/uploads/2023/03/Certifications-by-Year-
Subspecialties-2022.pdf. Accessed December 15, 2023.
742. Juul D, Colenda CC, Lyness JM, Dunn LB, Hargrave R, Faulkner
LR. Subspecialty training and certification in geriatric
psychiatry: a 25-year overview. Am Journal Geriatr Psych
2017;25(5):445-53.
743. Kozikowski A, Honda T, Segal-Gidan F, Hooker RS. Physician
assistants in geriatric medical care. BMC Geriatr 2020;20(1):1-8.
744. The Social Work Profession: Findings from Three Years of
Surveys of New Social Workers. Available at: https://www.cswe.
org/CSWE/media/Workforce-Study/The-Social-Work-
Profession-Findings-from-Three-Years-of-Surveys-of-New-
Social-Workers-Dec-2020.pdf. Accessed December 15, 2023.
745. Cummings S, Galambos C. Predictors of graduate social work
students’ interest in aging-related work. Journal of Gerontol
Soc Work 2002;39(3):77-94.
746. Cummings S, Adler G, DeCoster V. Factors influencing
graduate-social-work students’ interest in working with elders.
Educational Gerontology 2005;31(8):643-55.
747. Curl A, Simons K, Larkin H. Factors affecting willingness of
social work students to accept jobs in aging. J Soc Work
Education 2005;41(3):393-406.
748. Kolb P. Interest of racially and ethnically diverse social work
students in gerontological social work. Educational Gerontol
2008;34(10):907-22.
749. Ferguson A. Wanted: Gerontological social workers — Factors
related to interest in the field. Educational Gerontology
2012;38(10):713-28.
750. Ferguson A. The future of gerontological social work: what we
know and what we don't know about student interest in the
field. J Evid Inf Soc Work 2015;12(2):184-97.
138 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
751. Cortes T. "Estimate of geriatric specialization among RNs."
Received by Kezia Scales, November 14, 2022.
752. Orenstein S. Geriatric Nursing and Aging. Available at:
https://www.achca.org/index.php?option=com_
dailyplanetblog&view=entry&year=2020&month=03&day=
04&id=61:geriatric-nursing-and-aging. Accessed December
15, 2023.
753. Moye J, Karel MJ, Stamm KE, Qualls SH, Segal DL, Tazeau YN,
et al. Workforce Analysis of Psychological Practice With Older
Adults: Growing Crisis Requires Urgent Action. Train Educ Prof
Psychol 2019;13(1):46-55.
754. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S,
Forester BP. Emerging collaborative care models for dementia
care in the primary care setting: a narrative review. Am J
Geriatr Psychiatry 2020;28(3):320-30.
755. Frost R, Rait G, Aw S, Brunskill G, Wilcock J, Robinson L, Knapp
M, Hogan N, Harrison Dening K, Allan L, Manthorpe J.
Implementing post diagnostic dementia care in primary care: a
mixed-methods systematic review. Aging Ment Health
2021;25(8):1381-94.
756. Super N, Ahuja R, McDermott M. (2021). Scaling
Comprehensive Dementia-Care Models. Milken Institute.
Available at: https://milkeninstitute.org/sites/default/
files/2021-11/Comprehensive%20Dementia%20Care%20
Models.pdf. Accessed October 28, 2023.
757. French DD, LaMantia MA, Livin LR, Herceg D, Alder CA,
Boustani MA. Healthy Aging Brain Center improved care
coordination and produced net savings. Health Aff
2014;33(4):613-8.
758. Haggerty KL, Epstein-Lubow G, Spragens LH, Stoeckle RJ,
Evertson LC, Jennings LA, et al. Recommendations to improve
payment policies for comprehensive dementia care. J Am
Geriatr Soc 2020;68(11):2478-85.
759. Jennings LA, Laffan AM, Schlissel AC, Colligan E, Tan Z,
Wenger NS, et al. Health care utilization and cost outcomes of
a comprehensive dementia care program for Medicare
beneficiaries. JAMA Int Med 2019;179:161-6.
760. Possin KL, Merrilees JJ, Dulaney S, Bonasera SJ, Chiong W,
Lee K, et al. Effect of collaborative dementia care via telephone
and internet on quality of life, caregiver well-being, and health
care use: The Care Ecosystem Randomized Clinical Trial. JAMA
Intern Med 2019;179(12):1658-67.
761. Guterman EL, Kiekhofer RE, Wood AJ, Allen IE, Kahn JG,
Dulaneyet S. Care ecosystem collaborative model and health
care costs in Medicare beneficiaries with dementia: A
secondary analysis of a randomized clinical trial. JAMA Intern
Med 2023;183(11):1222-8.
762. Rosenbloom MH, Kashyap B, Diaz-Ochoa A, Karrmann J, Svitak
A, Finstad J, et al. Implementation and review of the care
ecosystem in an integrated healthcare system. BMC Geriatr
2023;23(1):515.
763. Care Ecosystem. Care Ecosystem Toolkit. Available at: https://
memory.ucsf.edu/sites/memory.ucsf.edu/files/wysiwyg/
CareEcosystemToolkit.pdf. Accessed December 15, 2023.
764. Scales K, Wagner LM. Direct care workers are the foundation
of a dementia-capable workforce. Generations 2023;47(1):1-9.
765. PHI. Direct Care Workers in the United States: Key Facts. New
York, NY: PHI, 2023. https://www.phinational.org/resource/
direct-care-workers-in-the-united-states-key-facts-2023/
Accessed September 18, 2023.
766. 766. Campbell S, Drake ADR, Espinoza R, Scales K. Caring for
the Future: The Power and Potential of America’s Direct Care
Workforce. Bronx, NY: PHI, 2021. https://www.phinational.org/
resource/caring-for-the-future-the-power-and-potential-of-
americas-direct-care-workforce/. Accessed December 15, 2023.
767. Reckrey JM, Tsui EK, Morrison RS, Geduldig ET, Stone RI,
Ornstein KA, et al. Beyond functional support: the range of
health-related tasks performed in the home by paid caregivers
in New York. Health Aff (Project Hope) 2019;38(6):927-33.
768. Lyons TL, Champion JD. Nonpharmacological interventions for
management of behavioral and psychological symptoms of
dementia in long-term care facilities by direct caregivers: A
systematic review. J Gerontol Nurs 2022;48(7):18-23.
769. Eaton J, Cloyes KG, Paulsen B, Madden C, Ellington L. The
development of knowledgeable nursing assistants as creative
caregivers (KNACC). Geriatr Nurs 2023;51:95-101.
770. Kemp CL, Bender AA, Morgan JC, Burgess EO, Epps FR, Hill
AM, Perkins MM. Understanding capacity and optimizing
meaningful engagement among persons living with dementia.
Dementia 2023;22(4):854-74.
771. Dokos M, Schultz R, Gossner JD, Fauth EB. Supporting persons
with dementia: perspectives from certified nurse’s assistants.
Innov Aging. 2023;7(5):igad049.
772. Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of
nursing home placement for older people with dementia: a
systematic review and meta-analysis. Int Psychogeriatr
2017;29(2):195-208.
773. Carnahan JL, Slaven JE, Callahan CM, Tu W, Torke AM.
Transitions From Skilled Nursing Facility to Home: The
Relationship of Early Outpatient Care to Hospital Readmission.
J Am Med Dir Assoc 2017;18(10):853-9.
774. Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-
Schwimmer EJ, et al. Transitional care interventions to prevent
readmissions for persons with heart failure: a systematic review
and meta-analysis. Ann Intern Med. 2014;160(11):774-84.
775. Murtaugh CM, Deb P, Zhu C, Peng TR, Barrón Y, Shah S, et al.
Reducing Readmissions among Heart Failure Patients
Discharged to Home Health Care: Effectiveness of Early and
Intensive Nursing Services and Early Physician Follow-Up.
Health Serv Res 2017;52(4):1445-72.
776. Guo W, Cai S, Caprio T, Schwartz L, Temkin-Greener H.
End-of-life care transitions in assisted living: associations with
state staffing and training regulations. J Am Med Dir Assoc
2023;24(6):827-32.
777. Shepherd H, Livingston G, Chan J, Sommerlad A. Hospitalisation
rates and predictors in people with dementia: a systematic
review and meta-analysis. BMC Med 2019;17(1):1-13.
778. LaMantia MA, Stump TE, Messina FC, Miller DK, Callahan CM.
Emergency department use among older adults with dementia.
Alzheimer Dis Assoc Disord 2016;30(1):35-40.
779. Yoon JM, Trinkoff AM, Kim M, Kim E. State-level nursing home
in-service dementia training requirements and inappropriate
psychotropic medication use. Geriatr Nursing 2023;51:209-14.
780. Centers for Medicare & Medicaid Services (CMS). Long-term
services and supports rebalancing toolkit. Available at: https://
www.medicaid.gov/medicaid/long-term-services-supports/
downloads/ltss-rebalancing-toolkit.pdf. Accessed December
15, 2023.
781. Shih RA, Friedman EM, Chen EK, Whiting GC. Prevalence and
correlates of gray market use for aging and dementia
long-term care in the US. J Appl Gerontol 2022; 41(4):1030-4.
782. Donlan A. After 3-Year Dip, Home Care Turnover Soars to 77%.
Home Health Care News, May 24, 2023. Available at: https://
homehealthcarenews.com/2023/05/after-dipping-for-three-
years-home-care-turnover-rate-soared-to-77-in-2022/.
Accessed September 18, 2023.
783. Gandhi A, Yu H, Grabowski DC. High nursing staff turnover in
nursing homes offers important quality information. Health Aff
2021;40(3):384-91.
784. Institute of Medicine. Retooling for an Aging America: Building
the Health Care Workforce. Washington, D.C.: The National
Academies Press 2008. Available at: http://www.
nationalacademies.org/hmd/reports/2008/retooling-for-an-
aging-america-building-the-health-care-workforce.aspx.
Accessed December 15, 2023.
785. Trinkoff AM, Han K, Storr CL, Lerner N, Johantgen M, Gartrell
K. Turnover, staffing, skill mix, and resident outcomes in a
national ample of US nursing homes. J Nurs Adm
2013;43(12):630-6.
786. Scales K. Transforming direct care jobs, reimagining long-term
services and supports. J Am Med Dir Assoc 2022;23(2):207-13.
787. Weller C, Almeida B, Cohen M, Stone R. Making Care Work
Pay. Available at: https://www.ltsscenter.org/wp-content/
uploads/2020/09/Making-Care-Work-Pay-Report-FINAL.pdf.
Accessed December 15, 2023.
788. Reckrey JM, Perez S, Watman D, Ornstein KA, Russell D,
Franzosa E. The need for stability in paid dementia care: family
caregiver perspectives. J Appl Gerontol 2023;42(4):607-16.
789. Manchha AV, Walker N, Way KA, Dawson D, Tann K, Thai M.
Deeply discrediting: A systematic review examining the
conceptualizations and consequences of the stigma of working
in aged care. Gerontologist 2021;61(4):e129-46.
790. Khavjou O, Suarez G, Tyler D, Squillace M, Dey J, Oliveira I.
Wages of direct care workers lower than other entry-level jobs
in most states (Issue brief). Washington, DC: Office of the
Assistant Secretary for Planning and Evaluation (ASPE), U.S.
Department of Health and Human Services. Available at:
https://aspe.hhs.gov/sites/default/files/
documents/7a611d901c615e5611ea095b1dcf8d08/
wages-dcw-lower-ib.pdf. Accessed September 18, 2023.
791. Burke G, Orlowski G. Training to serve people with dementia: is
our health care system ready? Available at: https://www.
justiceinaging.org/wp-content/uploads/2015/08/Training-to-
serve-people-with-dementia-Alz1_Final.pdf. Accessed
December 15, 2023.
792. U.S. Bureau of Labor Statistics (BLS). Occupational injuries and
illnesses and fatal injuries profiles. Available at: https://www.bls.
gov/iif/. Accessed December 15, 2023.
793. Paraprofessional Healthcare Institute (PHI). Workplace Injuries
and the Direct Care Workforce. Available at: https://
phinational.org/resource/workplace-injuries-direct-care-
workforce. Accessed December 15, 2023.
794. Quinn MM, Markkanen PK, Galligan CJ, Sama SR, Lindberg JE,
Edwards MF. Healthy aging requires a healthy home care
workforce: the occupational safety and health of home care
aides. Curr Environ Health Rep 2021;8(3):235-44.
795. Hebert CA, Scales K. Dementia friendly initiatives: A state of
the science review. Dementia 2019;18(5):1858-95.
796. Alzheimer’s Disease International. Dementia Friendly
Communities: Global Developments. 2016. Available at: https://
www.alzint.org/u/dfc-developments.pdf. Accessed December
15, 2023.
797. The White House. Fact sheet: The White House Conference on
Aging. Available at: https://obamawhitehouse.archives.gov/
the-press-office/2015/07/13/fact-sheet-white-house-
conference-aging. Accessed December 17, 2023.
798. ACT on Alzheimer's. About ACT on Alzheimer's. Available at:
https://actonalz.org/about. Accessed December 17, 2023.
799. Parke B, Boltz M, Hunter KF, Chambers T, Wolf-Ostermann K,
Adi MN, et al. A scoping literature review of dementia-friendly
hospital design. Gerontologist 2017;57(4):e62-e74.
800. Kirch J, Marquardt G. Towards human-centred general
hospitals: the potential of dementia-friendly design, Arch Sci
Rev 2023;66:5:382-90.
801. Riquelme-Galindo J, Lillo-Crespo M. Designing dementia care
pathways to transform non dementia-friendly hospitals:
Scoping review. Int J Enviro Res Public Health
2021;18(17):9296.
802. van Buuren LPG, Mohammadi M. Dementia-friendly design: A
Set of design criteria and design typologies supporting
wayfinding. HERD 2022;15(1):150-72.
803. Shiells K, Pivodic L, Holmerová I, Van den Block L. Self-
reported needs and experiences of people with dementia living
in nursing homes: a scoping review. Aging Ment Health
2020;24(10):1553-68.
804. Scher CJ, Somerville C, Greenfield EA, Coyle C. Organizational
characteristics of senior centers and engagement in dementia-
friendly communities. Innov Aging 2023;7(5);igad050.
805. Gan D, Chaudhury H, Mann J, Wister AV. Dementia-friendly
neighborhood and the built environment: A scoping review.
Gerontologist 2022;62(6):e340-e356.
806. Hung L, Hudson A, Gregorio M, Jackson L, Mann J, Horneet N.
Creating dementia-friendly communities for social inclusion: A
scoping review. Gerontol Geriatr Med
2021;7:23337214211013596.
807. Cherry DL. HCBS can keep people with dementia at home.
Generations 2012;36(1) 83-90.
808. Dickey TJ. "Public Libraries' Contribution to Sustainable
Dementia-Friendly Communities", Williams-Cockfield, K.C. and
Mehra, B. (Ed.) How Public Libraries Build Sustainable
Communities in the 21st Century (Advances in Librarianship, Vol.
53), Emerald Publishing Limited, Bingley, pp. 283-292. 2023.
809. Devos P, Aletta F, Thomas P, Petrovic M, Vander Mynsbrugge T,
Van de Velde D, et al. Designing supportive soundscapes for
nursing home residents with dementia. Int J Environ Res Public
Health 2019;16(24):4904.
810. Anetzberger GJ, Palmisano BR, Sanders M, Bass D, Dayton C,
Eckert S, Schimer MR. A model intervention for elder abuse
and dementia. Gerontologist 2000;40(4):492-7.
811. Dong XQ, Chen R, Simon MA. Elder abuse and dementia: A
review of the research and health policy. Health Aff (Millwood)
2014;33(4):642-9.
812. Sun F, Gao X, Brown H, Winfree LT. Police officer competence
in handling Alzheimer’s cases: The roles of AD knowledge,
beliefs, and exposure. Dementia. 2019;18(2):674-84.
813. Brown RT, Ahalt C, Rivera J, Stijacic Cenzer I, Wilhelm A,
Williams BA. Good cop, bad cop: Evaluation of a geriatrics training
program for police. J Am Geriatr Soc 2017;65(8):1842-7.
814. Anderson KA, Cimbal AM, Maile JJ. Hairstylists’ relationships
and helping behaviors with older adult clients. J Appl Gerontol
2010;29(3):371-80.
815. Che SL, Wu J, Chuang Y-C, Van IK, Leong SM. The willingness
to help people with dementia symptoms among four
occupation practitioners in Macao. Am J Alzheimers Dis Other
Demen 2022;37:15333175221139172.
816. Wang Q, Davis PB, Gurney ME, Xu R. COVID19 and dementia:
Analyses of risk, disparity, and outcomes from electronic health
records in the US. Alz Dement 2021;17(8):1297-306.
817. Health System Tracker. How has health sector employment
recovered since the pandemic? Available at: https://www.
healthsystemtracker.org/chart-collection/what-impact-has-
the-coronavirus-pandemic-had-on-healthcare-employment/.
Accessed December 15, 2023.
818. Wager E, Telesford I, Hughes-Cromwick P, Amin K, Cox C.
What impact has the coronavirus pandemic had on health
employment? Peterson-KFF Health System Tracker. Available
at: https://www.healthsystemtracker.org/chart-collection/
what-impact-has-the-coronavirus-pandemic-had-on-
healthcare-employment/#Cumulative%20percent%20
change%20in%20health%20sector%20and%20non-health%20
sector%20employment,%20January%201990-July%20
2022%C2%A0. Accessed December 15, 2023.
819. Centers for Medicare & Medicaid Services (CMS). COVID-19
nursing home data. Available at: https://data.cms.gov/covid-19/
covid-19-nursing-home-data. Accessed December 15, 2023.
820. Cutler DM. Challenges for the beleaguered health care
workforce during COVID-19. JAMA Health Forum 2022;3(1):
e220143.
821. Hendrickson RC, Slevin RA, Hoerster KD, Chang BP, Sano E,
McCall CA, et al. The impact of the COVID-19 pandemic on
mental health, occupational functioning, and professional
retention among health care workers and first responders. J
Gen Intern Med 2022;37(2):397-408.
822. White EM, Wetle TF, Reddy A, Baier RR. Front-line Nursing
Home Staff Experiences During the COVID-19 Pandemic. J
Am Med Dir Assoc. 2021;22(1):199-203. Erratum in: J Am Med
Dir Assoc. 2021 May;22(5):1123.
823. Office of the Surgeon General (OSG). Addressing health
worker burnout: The U.S. Surgeon General’s advisory on
building a thriving health workforce. Available at: https://www.
hhs.gov/sites/default/files/health-worker-wellbeing-advisory.
pdf. Accessed December 15, 2023.
824. Lluch C, Galiana L, Doménech P, Sansó N. The impact of the
COVID-19 pandemic on burnout, compassion fatigue, and
compassion satisfaction in healthcare personnel: A systematic
review of the literature published during the first year of the
pandemic. Healthcare 2022;10:364.
139
Appendices
140 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
825. Prasad K, McLoughlin C, Stillman M, Poplau S, Goelz E, Taylor S,
Nankivil N, Brown R, Linzer M, Cappelucci K, Barbouche M.
Prevalence and correlates of stress and burnout among US
healthcare workers during the COVID-19 pandemic: A national
cross-sectional survey study. eClinicalMedicine
2021;35:100879.
826. Kaufmann PG, Havens DS, Mensinger JL, Bradley PK, Brom
HM, Copel LC, et al. The COVID-19 study of healthcare and
support personnel (CHAMPS): Protocol for a longitudinal
observational study. JMIR Res Protocols 2021;10(10):e30757.
827. National Council on Aging. (2021, March 3). The impact of
COVID-19 on community-based organizations serving older
adults. Available at: https://www.ncoa.org/article/the-impact-
of-covid-19-on-community-based-organizations-serving-
older-adults. Accessed December 15, 2023.
828. Gallo HB, Wilber KH. Transforming aging services: Area
agencies on aging and the COVID-19 response. Gerontologist
2021;61(2):152-8.
829. Bourland DL, DeBacker L, Dotson Newman O, Loizeaux A.
Frontline organizations play a vital role in movement
ecosystems; let’s fund them to thrive. Am J Public Health
2022;112(1):63-5.
830. Numbers K, Brodaty H. The effects of the COVID-19 pandemic
on people with dementia. Nat Rev Neurol 2021 Feb;17(2):69-70.
831. American Public Health Association. Strengthening the
dementia care workforce: A public health priority. Available at:
https://www.apha.org/policies-and-advocacy/public-health-
policy-statements/policy-database/2021/01/13/
strengthening-the-dementia-care-workforce. Accessed
December 15, 2023.
832. Vespa J, Medina L, Armstrong DM. Demographic Turning
Points for the United States: Population Projections for 2020
to 2060. Current Population Reports. 2020 P25-1144, U.S.
Census Bureau, Washington, DC, 2020.
833. American Geriatrics Society (AGS). GWEP coordinating center.
Available at: https://www.americangeriatrics.org/programs/
gwep-coordinating-center. Accessed December 15, 2023.
834. National Institutes of Health. NIH RePorter: The National
Dementia Workforce Study. Available at: https://reporter.nih.
gov/search/tmkzdvxu9Uaz2nxxwrpybw/project-
details/10774551. Accessed December 15, 2023.
835. Galvin JE, Aisen P, Langbaum JB, Rodriguez E, Sabbagh M,
Stefanacci R, Stern RA, Vassey EA, de Wilde A, West N, Rubino
I. Early stages of Alzheimer's disease: Evolving the care team for
optimal patient management. Front Neurol 2021;11:592302.
836. Lassell RK, Moreines LT, Luebke MR, Bhatti KS, Pain KJ, Brody
AA, Luth EA. Hospice interventions for persons living with
dementia, family members and clinicians: A systematic review. J
Am Geriatr Soc 2022;70(7):2134-45.
837. Alzheimer’s Association. Alzheimer’s facts and figures 2021
special report: Race, ethnicity and Alzheimer's in America.
Available online https://www.alz.org/media/Documents/
alzheimers-facts-and-figures-special-report-2021.pdf.
Accessed December 15, 2023.
838. Díaz-Santos M, Yáñez J, Suarez PA. Alzheimer’s disease in
bilingual Latinos: clinical decisions for diagnosis and treatment
planning. J Health Service Psychol 2021;47(4):171-9.
839. Chejor P, Laging B, Whitehead L, Porock D. Experiences of
older immigrants living with dementia and their carers: a
systematic review and meta-synthesis. BMJ open
2022;12(5):e059783.
840. Nowaskie DZ, Sewell, DD. Assessing the LGBT cultural
competency of dementia care providers. Alzheimers Dement
2021;7(1): e12137.
841. Eller N, Belza B. Addressing Alzheimer’s disease in Asian
American and Pacific Islander older adults: An action guide for
service providers. J Gerontol Nurs 2019;44(4):3-4.
842. Abramsohn EM, Paradise KM, Glover CM, Benjamins M,
Douglas L, Jerome J, et al. CommunityRx: Optimizing a
community resource referral intervention for minority
dementia caregivers. J Appl Gerontol 2022;41(1):113-23.
843. Alzheimer’s Association. The Alzheimer’s and Dementia Care
ECHO® Program for clinicians. Available at: https://www.alz.
org/professionals/health-systems-clinicians/echo-alzheimers-
dementia-care-program. Accessed December 15, 2023.
844. Dementia Care Aware. Early Detection. Better Care. Available
at: https://www.dementiacareaware.org/. Accessed December
15, 2023.
845. Medi-Cal. Medi-Cal Rates. Available at: https://mcweb.apps.prd.
cammis.medi-cal.ca.gov/rates?page=1&tab=rates. Accessed
December 15, 2023.
846. Dementia Care Aware. 2023. Guide to Cognitive Impairment
Screening and Billing in California. Available at: https://www.
dementiacareaware.org/wp-content/uploads/2023/11/
Dementia-Care-Aware_Guide-to-Cognitive-Impairment-
Screening-and-Billing-in-California.pdf. Accessed December
16, 2023.
847. The Gerontological Society of America. The GSA KAER Toolkit
for Primary Care Teams: Supporting Conversations about Brain
Health, Timely Detection of Cognitive Impairment, and
Accurate Diagnosis of Dementia. Fall 2020 Edition. Available at:
https://www.geron.org/images/gsa/Marketing/KAER/GSA_
KAER-Toolkit_2020_Final.pdf. Accessed December 15, 2023.
848. Poghosyan L, Brooks JM, Hovsepian V, Pollifrone M, Schlak AE,
Sadak T. The growing primary care nurse practitioner
workforce: A solution for the aging population living with
dementia. Am J Geriatr Psychiatry 2021;29(6):517-26.
849. Gaps in the Dementia Care Workforce: Research Update and
Data Needs. Committee on Population (CPOP) Semi-Annual
Meeting, May 23, 2019. Available at: https://www.nia.nih.gov/
sites/default/files/2019-11/Seminar-Gaps-Dementia-
Workforce-Final-508.pdf. Accessed December 15, 2023.
850. Bonner A, Walsh A, Shue J, Morton G, Fulmer T. (2023).
Generations: Dementia-Friendly Initiatives for Individuals
Living with Dementia, Care Partners, and Communities.
Available at: https://generations.asaging.org/dementia-
friendly-initiatives. Accessed October 28, 2023.
851. Ty D, McDermott M. (2021). Building Workforce Capacity to
Improve Detection and Diagnosis of Dementia. Milken Institute.
Available at: https://milkeninstitute.org/sites/default/
files/2021-05/Building%20Dementia%20Workforce.pdf.
Accessed October 28, 2023.
852. Boustani M, Alder CA, Solid CA, Reuben D. An alternative
payment model to support widespread use of collaborative
dementia care models. Health Aff (Millwood) 2019;38(1):54-9.
853. Pizzi LT, Jutkowitz E, Prioli KM, Lu E, Babcock Z, McAbee-
Sevick H, Wakefield DB, Robison J, Molony S, Piersol CV, Gitlin
LN. Cost–benefit analysis of the COPE program for persons
living with dementia: Toward a payment model. Innov Aging
2022;6(1):igab042.
854. Li J, Andy C, Mitchell S. Use of Medicare’s new reimbursement
codes for cognitive assessment and care planning, 2017-2018.
JAMA Netw Open 2021;4(9):e2125725.
855. U.S. Centers for Medicare & Medicaid. Guiding an improved
dementia experience (GUIDE) model. Available at: https://www.
cms.gov/priorities/innovation/innovation-models/guide.
Accessed December 15, 2023.
856. Winters A, Block L, Maxey H, Medlock C, Ruane K,
Hockenberry S. State Strategies for Sector Growth and
Retention for the Direct Care Health Workforce. 2021
Washington, DC: National Governors Association Center for
Best Practices. Available at: https://www.nga.org/wp-content/
uploads/2021/10/NGA_SectorGrowth-DirectCare_report.
pdf. Accessed December 15, 2023.
857. Scales K. State policy strategies for strengthening the direct
care workforce. Available at: https://www.phinational.org/
resource/state-policy-strategies-for-strengthening-the-
direct-care-workforce/. Accessed December 15, 2023.
858. Muirhead K, Macaden L, Smyth K, Chandler C, Clarke C, Polson
R, O’Malley C. Establishing the effectiveness of technology-
enabled dementia education for health and social care
practitioners: a systematic review. Syst Rev 2021;10(1):1-26.
859. Geddes MR, O'Connell ME, Fisk JD, Gauthier S, Camicioli R,
Ismail Z. Remote cognitive and behavioral assessment: Report
of the Alzheimer Society of Canada Task Force on Dementia
Care Best Practices for COVID-19. Alzheimers Dement
2020;12(1):e12111.
860. Yi JS, Pittman CA, Price CL, Nieman CL, Oh ES. Telemedicine
and dementia care: a systematic review of barriers and
facilitators. J Am Med Dir Assoc 2021;22(7):1396-1402.
861. Pappadà A, Chattat R, Chirico I, Valente M, Ottoboni G.
Assistive technologies in dementia care: an updated analysis of
the literature. Front Psych 2021;12:644587.
862. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM.
Monetary costs of dementia in the United States. N Engl J Med
2013;368:1326-34.
863. Unpublished tabulations based on data from the Medicare
Current Beneficiary Survey for 2018. Prepared under contract
by Health Care Cost Institute, December 2021.
864. Yang Z, Zhang K, Lin PJ, Clevenger C, Atherly A. A longitudinal
analysis of the lifetime cost of dementia. Health Serv Res
2012;47(4):1660-78.
865. Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda
CC. The incremental direct costs associated with behavioral
symptoms in AD. Neurology 2022;59:1721-9.
866. Yang Z, Levey A. Gender differences: A lifetime analysis of the
economic burden of Alzheimer’s disease. Women Health Iss
2015;25(5):436-40.
867. White L, Fishman P, Basu A, Crane PK, Larson EB, Coe NB.
Medicare expenditures attributable to dementia. Health
Services Res 2019;54(4):773-81.
868. Coe NB, White L, Oney M, Basu A, Larson EB. Public spending
on acute and long-term care for Alzheimer's disease and
related dementias. Alzheimers Dement 2023;19(1):150-7.
869. Oney M, White L, Coe NB. Out-of-pocket costs attributable to
dementia: A longitudinal analysis. J Am Geriatr Soc
2022;70(5):1538-45.
870. Hudomiet P, Hurd MD, Rohwedder S. The relationship between
lifetime out-of-pocket medical expenditures, dementia and
socioeconomic status in the U.S. J Econ Ageing
2019;14:100181.
871. Dwibedi N, Findley AP, Wiener C, Shen C, Sambamoorthi U.
Alzheimer disease and related disorders and out-of-pocket
health care spending and burden among elderly Medicare
beneficiaries. Medical Care 2018;56:240-6.
872. Leniz J, Yi D, Yorganci E, Williamson LE, Suji T, Cripps R, et al.
Exploring costs, cost components, and associated factors
among people with dementia approaching the end of life: A
systematic review. Alzheimers Dement (NY) 2021;7(1):e12198.
873. Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of
health care costs for patients with dementia in the last 5 years
of life. Ann Intern Med 2015;163:729-36.
874. Rudolph JL, Zanin NM, Jones RN, Marcantonio ER, Fong TG,
Yang FM, et al. Hospitalization in community-dwelling persons
with Alzheimer’s disease: Frequency and causes. J Am Geriatr
Soc 2010;58(8):1542-8.
875. Beydoun MA, Beydoun HA, Gamaldo AA, Rostant O, Dore GA,
Zonderman AB, et al. Nationwide inpatient prevalence,
predictors and outcomes of Alzheimer’s disease among older
adults in the United States, 2002–2012. J Alzheimers Dis
2015;48(2):361-75.
876. Landon BE, Keating NL, Onnela JP, Zaslavsky AM, Christakis
NA, O’Malley AJ. Patient-sharing networks of physicians and
health care utilization and spending among Medicare
beneficiaries. JAMA Intern Med 2018;178:66-73.
877. U.S. Centers for Medicare & Medicaid Services. State Level
Chronic Conditions Table: Prevalence, Medicare Utilization and
Spending, 2007-2018. Available at: https://www.cms.gov/
Research-Statistics-Data-and-Systems/Statistics-Trends-and-
Reports/Chronic-Conditions/CC_Main.html. Accessed
December 15, 2023.
878. Cairns C, Kang K. National Hospital Ambulatory Medical Care
Survey: 2021 emergency department summary tables.
Available from: https://ftp.cdc.gov/pub/Health_Statistics/
NCHS/ Dataset_Documentation/NHAMCS/doc21-ed-508.pdf.
Accessed October 27, 2023.
879. Hill JD, Schmucker AM, Siman N, Goldfeld KS, Cuthel AM,
Chodosh J, et al. Emergency and post-emergency care of older
adults with Alzheimer's disease/Alzheimer's disease related
dementias. J Am Geriatr Soc 2022;70(9):2582-91.
880. Medicare. Glossary. Medicare: The Official U.S. Government
Site for Medicare. Available at: https://www.medicare.gov/
glossary/a. Accessed December 15, 2023.
881. U.S. Centers for Medicare & Medicaid Services. Mapping
Medicare disparities by population. Available at: https://data.
cms.gov/tools/mapping-medicare-disparities-by-population.
Accessed December 12, 2023.
882. Leibson CL, Hall Lon K, Ransom JE, Roberts RO, Hass SL,
Duhig AM, et al. Direct medical costs and source of cost
differences across the spectrum of cognitive decline: A
population-based study. Alzheimers Dement 2015;11(8):917-32.
883. Suehs BT, Davis CD, Alvir J, van Amerongen D, Patel NC, Joshi
AV, et al. The clinical and economic burden of newly diagnosed
Alzheimer’s disease in a Medicare Advantage population. Am J
Alzheimers Dis Other Dement 2013;28(4):384-92.
884. Lin P-J, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare
expenditures of individuals with Alzheimer’s disease and related
dementias or mild cognitive impairment before and after
diagnosis. J Am Geriatr Soc 2016;64:1549-57.
885. Geldmacher DS, Kirson NY, Birnbaum HG, Eapen S, Kantor E,
Cummings AK, et al. Pre-diagnosis excess acute care costs in
Alzheimer’s patients among a U.S. Medicaid population. Appl
Health Econ Health Policy 2013;11(4):407-13.
886. Zhu CW, Cosentino S, Ornstein K, Gu Y, Scarmeas N, Andrews
H, et al. Medicare utilization and expenditures around incident
dementia in a multiethnic cohort. J Gerontol A Biol Sci Med Sci
2015;70(11):1448-53.
887. Kirson NY, Desai U, Ristovska L, Cummings AKG, Birnbaum HG,
Ye W, et al. Assessing the economic burden of Alzheimer’s
disease patients first diagnosed by specialists. BMC Geriatrics
2016;16:138.
888. Aigbogun MS, Stellhorn R, Hartry A, Baker RA, Fillit H.
Treatment patterns and burden of behavioral disturbances in
patients with dementia in the United States: A claims database
analysis. BMC Neurology 2019;19:33.
889. Davis-Ajami ML, Lu ZK, Wu J. Exploring the home healthcare
workforce in Alzheimer’s disease and related dementias:
Utilization and cost outcomes in US community dwelling older
adults. Arch Gerontol Geriat 2022;98;104536.
890. Ochieng N, Fuglesten Biniek J, Freed M, Damico A, Neuman T.
Medicare Advantage in 2023: Premiums, out-of-pocket limits,
cost sharing, supplemental benefits, prior authorization and
star ratings. Kaiser Family Foundation. Available at: https://
www.kff.org/medicare/issue-brief/medicare-advantage-in-
2023-premiums-out-of-pocket-limits-cost-sharing-
supplemental-benefits-prior-authorization-and-star-ratings/.
Accessed November 15, 2023.
891. U.S. Centers for Medicare & Medicaid Services. SNP
Comprehensive Report - November 2023. Available at: https://
www.cms.gov/research-statistics-data-and-systems/statistics-
trends-and-reports/mcradvpartdenroldata/special/snp-
comprehensive-report-2023-11. Accessed November 15, 2023.
892. U.S. Centers for Medicare & Medicaid Services. National Health
Expenditures Tables: Table 12 Expenditures, Enrollment and
Per Enrollee Estimates of Health Insurance. Last modified
September 6, 2023. Available at: https://www.cms.gov/
data-research/statistics-trends-and-reports/national-health-
expenditure-data/nhe-fact-sheet. Accessed November 15,
2023.
893. National Center for Health Statistics. Biennial Overview
of Post-acute and Long-term Care in the United States.
Available at: https://data.cdc.gov/d/wibz-pb5q. Accessed
November 25, 2023.
141
Appendices
142 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
894. Knox S, Downer B, Haas A, Ottenbacher KJ. Home health
utilization association with discharge to community for people
with dementia. Alzheimers Dement (NY) 2022;8(1):e12341.
895. Rome V, Penn Lendon J, Harris-Kojetin L. Differences in
characteristics of adult day services centers by level of medical
service provision. National Center for Health Statistics
2020;3(45):1-28.
896. Sengupta M, Caffrey C. Characteristics of residential care
communities by percentage of resident population diagnosed
with dementia: United States, 2016. National Health Statistics
Reports 2020;148:1-7.
897. Caffrey C, Melekin A, Lu Z, Sengupta M. Variation in residential
care community resident characteristics, by size of community:
United States, 2020. NCHS Data Brief, no 454. Hyattsville, MD:
National Center for Health Statistics. 2022.
898. Caffrey C, Harris-Kojetin L, Rome V, Sengupta M. Variation in
operating characteristics of residential care communities by
size of community: United States, 2014. NCHS Data Brief, No.
222. November 2015.
899. Colelo KJ. Who pays for long-term services and supports?
Congressional Research Service, In Focus, IF10343. August 5,
2021. Available at: https://crsreports.congress.gov/. Accessed
December 15, 2023.
900. Murray C, Eckstein M, Lipson D, Wysocki A. Medicaid Long Term
Services and Supports Annual Expenditures Report: Federal
Fiscal Year 2020. Chicago, IL: Mathematica, June 9, 2023.
901. McGarry BE, Grabowski DC. Medicaid home and community-
based services spending for older adults: Is there a "woodwork"
effect? J Am Geriatr Soc. 2023;71(10):3143-51.
902. Bynum J. Characteristics, Costs, and Health Service Use for
Medicare Beneficiaries with a Dementia Diagnosis: Report 1:
Medicare Current Beneficiary Survey. Unpublished; provided
under contract with the Alzheimer’s Association. Lebanon,
N.H.: Dartmouth Institute for Health Policy and Clinical Care,
Center for Health Policy Research, January 2009.
903. Clarkson P, Davies L, Jasper R, Loynes N, Challis D. Home
Support in Dementia (HoSt-D) Programme Management
Group. A systematic review of the economic evidence for home
support interventions in dementia. Value in Health
2017;20:1198-209.
904. Nickel F, Barth J, Kolominsky-Rabas PL. Health economic
evaluations of non-pharmacological interventions for persons
with dementia and their informal caregivers: A systematic
review. BMC Geriatrics 2018;18:69.
905. Callahan CM, Arling G, Tu W, Rosenman MB, Counsell SR,
Stump TE, et al. Transitions in care among older adults with and
without dementia. J Am Geriatr Soc 2012;60(5):813-20.
906. Gozalo P, Teno JM, Mitchell SL, Skinner J, Bynum J, Tyler D,
et al. End-of-life transitions among nursing home residents
with cognitive issues. N Engl J Med 2011;365(13):1212-21.
907. Teno JM, Mitchell SL, Skinner J, Kuo S, Fisher E, Intrator O,
et al. Churning: The association between health care transitions
and feeding tube insertion for nursing home residents with
advanced cognitive impairment. J Palliat Med 2009;12(4):359-62.
908. Genworth. Genworth Cost of Care Survey. Genworth Financial,
Inc. Available at: https://www.genworth.com/aging-and-you/
finances/cost-of-care.html. Accessed December 15, 2023.
909. Unpublished data from the 2018 Medicare Current Beneficiary
Survey (MCBS), analyzed by the Alzheimer's Association.
October 2020.
910. Koma W, Neuman T, Jacobson G, Smith K. Medicare
beneficiaries’ financial security before the coronavirus
pandemic. Issue Brief. Kaiser Family Foundation. www.kff.org/
medicare/issue-brief/medicare-beneficiaries-financial-
security-before-the-coronavirus-pandemic/. Accessed on
December 15, 2023.
911. Hamel L, Montero A. The affordability of long-term care and
support services: Findings from a KFF survey. Kaiser Family
Foundation. November 14, 2023. Available at: https://www.kff.
org/health-costs/poll-finding/the-affordability-of-long-term-
care-and-support-services/. Accessed November 25, 2023.
912. U.S. Centers for Medicare & Medicaid Services. Your Medicare
Coverage. Long-Term Care. Available at: https://www.medicare.
gov/coverage/long-term-care.html. Accessed December 15, 2023.
913. National Association of Insurance Commissioners and the
Center for Insurance Policy and Research. The State of
Long-Term Care Insurance: The Market, Challenges and Future
Innovations. CIPR Study Series 2016-1. May 2016.
914. U.S. Centers for Medicare & Medicaid Services. Skilled nursing
facility (SNF) care. https://www.medicare.gov/coverage/
skilled-nursing-facility-snf-care. Accessed December 15, 2023.
915. U.S. Centers for Medicare & Medicaid Services. 2024 Medicare
Parts A and B premiums and deductibles. October 23, 2023.
Available at: https://www.cms.gov/newsroom/fact-
sheets/2024-medicare-parts-b-premiums-and-deductibles?ce
id=9903174&emci=19d1679d-e769-ee11-9937-
00224832eb73&emdi=fa03f56d-f869-ee11-9937-
00224832eb73. Accessed December 1, 2023.
916. U.S. Centers for Medicare & Medicaid Services. What Are
Long-Term Care Hospitals? CMS Product No. 11347. https://
www.medicare.gov/Pubs/pdf/11347-Long-Term-Care-
Hospitals.pdf. Revised June 2019. Accessed December 15, 2023.
917. U.S. Centers for Medicare & Medicaid Services. Original
Medicare (Part A and B) Eligibility and Enrollment. https://www.
cms.gov/Medicare/Eligibility-and-Enrollment/
OrigMedicarePartABEligEnrol. Accessed December 15, 2023.
918. Ochieng N, Fuglesten Biniek J, Freed M, Damico A, Neuman T.
Medicare Advantage in 2023: Enrollment update and key
trends. Kaiser Family Foundation. August 9, 2023. Available at:
https://www.kff.org/medicare/issue-brief/medicare-
advantage-in-2023-enrollment-update-and-key-trends/.
Accessed November 25, 2023.
919. U.S. Centers for Medicare & Medicaid Services. How Do
Medicare Advantage Plans Work? https://www.medicare.gov/
sign-up-change-plans/types-of-medicare-health-plans/
medicare-advantage-plans/how-do-medicare-advantage-
plans-work. Accessed December 15, 2023.
920. U.S. Centers for Medicare & Medicaid Services. What’s
Medicare? What’s Medicaid? CMS Product No. 11306. https://
www.medicare.gov/Pubs/pdf/11306-Medicare-Medicaid.pdf.
Accessed December 15, 2023.
921. U.S. Department of Health and Human Services. What is
Long-Term Care Insurance? Available at: https://acl.gov/ltc/
costs-and-who-pays/what-is-long-term-care-insurance.
Accessed December 15, 2023.
922. American Association for Long-Term Care Insurance.
Long-term care insurance facts - data - statistics - 2022
reports. Available at: https://www.aaltci.org/long-term-care-
insurance/learning-center/ltcfacts-2022.php. Accessed
November 15, 2023.
923. AHIP. Long-term care insurance coverage: State-to-state
2023. Available at: https://www.ahip.org/documents/AHIP_
LTC_State_Data_Report.pdf. Accessed November 15, 2023.
924. U.S. Department of the Treasury. Long-Term Care Insurance:
Recommendations for Improvement of Regulation. Report of
the Federal Interagency Task Force on Long-Term Care
Insurance. August 2020. Available at: https://home.treasury.gov/
system/files/136/Report-Federal-Interagency-Task-Force-
Long-Term-Care-Insurance.pdf. Accessed December 16, 2023.
925. Washington State Legislature. Chapter 50B.04 RCW. Long-
Term Services and Supports Program. https://app.leg.wa.gov/
RCW/default.aspx?cite=50B.04. Accessed December 15, 2023.
926. Washington State Department of Social and Health Services.
About the WA Cares Fund. https://wacaresfund.wa.gov/
about-the-wa-cares-fund/. Accessed December 15, 2023.
927. LTCNews. Multiple states considering implementing long-term
care tax. Updated July 22, 2023. Available at: https://www.ltcnews.
com/articles/multiple-states-considering-implementing-long-
term-care-tax. Accessed November 15, 2023.
928. U.S. Centers for Medicare & Medicaid Services. Seniors &
Medicare and Medicaid enrollees. Available at: https://www.
medicaid.gov/medicaid/eligibility/seniors-medicare-and-
medicaid-enrollees/index.html. Accessed December 15, 2023.
929. U.S. Centers for Medicare & Medicaid Services. Medicare and
hospice benefits: Getting Started. Care and support for people
who are terminally ill. CMS Product No. 11361. Revised March
2020. Available at www.medicare.gov/Pubs/pdf/11361-
Medicare-Hospice-Getting-Started.pdf. Accessed December
15, 2023.
930. De Vleminck A, Morrison RS, Meier DE, Aldridge MD. Hospice
care for patients with dementia in the United States: A
longitudinal cohort study. J Am Med Dir Assoc 2018;19:633-8.
931. U.S. Centers for Medicare & Medicaid Services. Post-Acute
Care and Hospice Provider Data 2017. Available at: https://
www.hhs.gov/guidance/document/post-acute-care-and-
hospice-provider-data-0. Accessed December 15, 2023.
932. Davis MA, Chang C-H, Simonton, S, Bynum J. 2022. Trends in
US Medicare decedents' diagnosis of dementia from 2004 to
2017. JAMA Health Forum 2022;3(4):e220346.
933. Aldridge MD, Hunt L, Husain M, Li L, Kelley A. Impact of
Comorbid Dementia on Patterns of Hospice Use. J Palliat Med
2022;25(3):396-404.
934. Russell D, Diamond EL, Lauder B, Digham RR, Dowding DW,
Peng TR, et al. Frequency and risk factors for live discharge
from hospice. J Am Geriatr Soc. 2017;65:1726-32.
935. U.S. Department of Health and Human Services. U.S. Centers
for Medicare & Medicaid Services. CMS Manual System.
Medicare Program; FY 2024 Hospice Wage Index and Payment
Rate Update, Hospice Conditions of Participation Updates,
Hospice Quality Reporting Program Requirements, and
Hospice Certifying Physician Provider Enrollment
Requirements. Document Number 2023-16116. Available at:
https://www.federalregister.gov/
documents/2023/08/02/2023-16116/medicare-program-fy-
2024-hospice-wage-index-and-payment-rate-update-
hospice-conditions-of. Accessed November 10, 2023.
936. National Archives. Code of Federal Regulations (eCFR).
Certification of terminal illness. Available at: https://www.ecfr.
gov/current/title-42/chapter-IV/subchapter-B/part-418/
subpart-B/section-418.22. Accessed December 15, 2023.
937. Gozalo P, Plotzke M, Mor V, Miller SC, Teno JM. Changes in
Medicare costs with the growth of hospice care in nursing
homes. N Engl J Med 2015;372:1823-31.
938. Miller SC, Lima JC, Looze J, Mitchell SL. Dying in U.S. nursing
homes with advanced dementia: How does health care use
differ for residents with, versus without, end-of-life Medicare
skilled nursing facility care? J Palliat Med 2012;15:43-50.
939. Miller SC, Gozalo P, Mor V. Hospice enrollment and
hospitalization of dying nursing home patients. Am J Med
2001;11(1):38-44.
940. Kiely DK, Givens JL, Shaffer ML, Teno JM, Mitchell SL. Hospice
use and outcomes in nursing home residents with advanced
dementia. J Am Geriatr Soc 2010;58(12):2284-91.
941. Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs
GA. Patients dying with dementia: Experience at the end of life
and impact of hospice care. J Pain Symptom Manage
2008;35(5):499-507.
942. Lin PJ, Zhu Y, Olchanski N, Cohen JT, Neumann PJ, Faul JD,
et al. Racial and ethnic differences in hospice use and
hospitalizations at end-of-life among medicare beneficiaries
with dementia. JAMA Netw Open 2022;5(6):e2216260.
943. Miller SC, Lima JC, Orna I, Martin E, Bull J. Hanson LC. Specialty
palliative care consultations for nursing home residents with
dementia. J Pain Symptom Manage 2017;54:9-16.
944. Palmer MK, Jacobson M, Enguidanos S. Advance care planning
for Medicare beneficiaries increased substantially, but
prevalence remained low. Health Aff 2021;40:613-21.
945. Zhu Y, Olchanski N, Cohen JT, Freund KM, Faul JD, Fillit HM,
Neumann PJ, Lin PJ. Life-sustaining treatments among
Medicare beneficiaries with and without dementia at the end of
life. J Alzheimers Dis 2023;96(3):1183-93.
946. Bynum JPW, Meara E, Chang C-H, Rhoads JM. Our Parents,
Ourselves: Health Care for an Aging Population. A Report of
the Dartmouth Atlas Project. The Dartmouth Institute for
Health Policy & Clinical Practice; 2016.
947. Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube
feeding in U.S. nursing home residents with advanced
dementia, 2000-2014. JAMA 2016;316(7):769-70.
948. Centers for Disease Control and Prevention, National Center
for Health Statistics. National Vital Statistics System, Mortality
1999-2020 on CDC WONDER Online Database, released in
2021. Data are from the Multiple Cause of Death Files,
1999-2020, as compiled from data provided by the 57 vital
statistics jurisdictions through the Vital Statistics Cooperative
Program. Available at: http://wonder.cdc.gov/ucd-icd10.html.
Accessed December 12, 2023.
949. Centers for Disease Control and Prevention, National Center
for Health Statistics. National Vital Statistics System, Mortality
2018-2021 on CDC WONDER Online Database, released in
2021. Data are from the Multiple Cause of Death Files,
2018-2021, as compiled from data provided by the 57 vital
statistics jurisdictions through the Vital Statistics Cooperative
Program. Available at: http://wonder.cdc.gov/ucd-icd10-
expanded.html. Accessed December 12, 2023.
950. Park S, Chen J. Racial and ethnic patterns and differences in
health care expenditures among Medicare beneficiaries with
and without cognitive deficits or Alzheimer’s disease and
related dementias. BMC Geriatrics 2020;20:482.
951. Gilligan AM, Malone DC, Warholak TL, Armstrong EP. Health
disparities in cost of care in patients with Alzheimer’s disease:
An analysis across 4 state Medicaid populations. Am J
Alzheimers Dis Other Dement 2013;28(1):84-92.
952. Lin P-J, Zhong Y, Fillit HM, Cohen JT, Neumann PJ.
Hospitalizations for ambulatory care sensitive conditions and
unplanned readmissions among Medicare beneficiaries with
Alzheimer’s disease. Alzheimers Dement 2017;13(10):1174-8.
953. U.S. Department of Health and Human Services. Reduce the
proportion of preventable hospitalizations in older adults with
dementia — DIA-02. Healthy People 2030. Available at:
https://health.gov/healthypeople/objectives-and-data/
browse-objectives/dementias/reduce-proportion-
preventable-hospitalizations-older-adults-dementia-dia-02.
Accessed December 15, 2023.
954. Davydow DS, Zibin K, Katon WJ, Pontone GM, Chwastiak L,
Langa KM, et al. Neuropsychiatric disorders and potentially
preventable hospitalizations in a prospective cohort study of
older Americans. J Gen Intern Med 2014;29(10):1362-71.
955. Guterman EL, Allen IE, Josephson SA, Merrilees JJ, Dulaney S,
Chiong W, et al. Association between caregiver depression and
emergency department use among patients with dementia.
JAMA Neurol 2019;76:1166-73.
956. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski
EZ. Mini-Cog performance: Novel marker of post discharge risk
among patients hospitalized for heart failure. Circ Heart Fail
2015;8(1):8-16.
957. Lin PJ, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable
hospitalizations among Medicare beneficiaries with Alzheimer’s
disease and related disorders. Alzheimers Dement 2013;9(1):30-8.
958. MacNeil-Vroomen JL, Nagurney JM, Allore HG. Comorbid
conditions and emergency department treat and release
utilization in multimorbid persons with cognitive impairment.
Am J Emerg Med 2020;38(1):127-31.
959. Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use
among Medicare beneficiaries with dementia varies by setting
and proximity to death. Health Aff 2014;33(4):683-90.
960. White EM, Kosar CM, Rahman M, Mor V. Trends in hospitals and
skilled nursing facilities sharing medical providers. Health
Affairs 2020;39(8):1312-20.
961. Chidambaram P, Burns A. Ten things about long-term services
and supports (LTSS). Kaiser Family Foundation. November 16,
2023. Available at: https://www.kff.org/medicaid/issue-
brief/10-things-about-long-term-services-and-supports-ltss/
Accessed November 25, 2023.
962. U.S. Centers for Medicare & Medicaid Services. Medicare
COVID-19 cases & hospitalizations. January 2022. Available at:
https://data.cms.gov/covid-19/medicare-covid-19-cases-
hospitalizations/data. Accessed December 15, 2023.
143
Appendices
144 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
963. Lamont H, Samson LW, Zuckerman R, Dey J, Oliveira I, Tarazi
W. The Impact of COVID-19 on Medicare Beneficiaries with
Dementia (Issue Brief). Washington, DC: Office of the Assistant
Secretary for Planning and Evaluation, U.S. Department of
Health and Human Services. April 6, 2021.
964. Wang Q, Davis PB, Gurney ME, Xu R. COVID-19 and dementia:
Analyses of risk, disparity, and outcomes from electronic health
records in the US. Alzheimers Dement 2021;17(8):1297-1306.
965. Centers for Medicare & Medicaid Services. The Impact
of COVID-19 on Medicare Beneficiaries in Nursing Homes.
Available at: https://www.cms.gov/files/document/medicare-
covid-19-nursing-home-analysis.pdf. Accessed December 15, 2023.
966. Cubanski J, Neuman T. FAQs on Medicare Financing and Trust
Fund Solvency. Kaiser Family Foundation, March 16, 2021.
Available at: https://www.kff.org/medicare/issue-brief/
faqs-on-medicare-financing-and-trust-fund-solvency/.
Accessed December 15, 2023.
967. Burke JF, Kerber KA, Langa KM, Albin RL, Kotagal V.
Lecanemab: Looking before we leap. Neurology
2023;101(15):661-5.
968. Rosenthal MB. Novel Alzheimer Disease Treatments and
Reconsideration of US Pharmaceutical Reimbursement Policy.
JAMA 2023;330(6):505-6.
969. Global Eisai. Eisai’s Approach to U.S. Pricing for Leqembi
(Lecanemab), a Treatment for Early Alzheimer’s Disease, Sets
Forth Our Concept of “Societal Value of Medicine” in Relation
to “Price of Medicine.” Published January 7, 2023. Available at:
https://www.eisai.com/news/2023/news202302.html. Accessed
January 31, 2024.
970. Mahase E. Alzheimer's disease: FDA approves lecanemab amid
cost and safety concerns. BMJ 2023;380:73.
971. U.S. Centers for Medicare & Medicaid Services. CMS announces
new details of plan to cover new Alzheimer’s drugs. Published
June 22, 2023. Available at: https://www.cms.gov/newsroom/
fact-sheets/cms-announces-new-details-plan-cover-new-
alzheimers-drugs. Accessed November 10, 2023.
972. Pittock RR, Aakre J, Castillo AM, Ramanan VK, Kremers WK,
Jack CR, et al. Eligibility for Anti-Amyloid Treatment in a
Population-Based Study of Cognitive Aging. Neurology
2023;101(19):e1837-e1849.
973. Alzheimer’s Association. Changing the Trajectory of Alzheimer’s
Disease: How a Treatment by 2025 Saves Lives and Dollars.
Available at: https://www.alz.org/help-support/resources/
publications/trajectory_report. Accessed December 15, 2023.
974. Zissimopoulos J, Crimmins E, St. Clair P. The value of delaying
Alzheimer’s disease onset. Forum Health Econ Policy.
2014;18(1):25-39.
975. Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and
Figures. Alzheimers Dement 2018;14(3):408-11.
976. Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the
Preparedness of the U.S. Health Care System Infrastructure for
an Alzheimer's Treatment. Santa Monica, CA: RAND
Corporation, 2017. Available at: https://www.rand.org/pubs/
research_reports/RR2272.html. Accessed December 15, 2023.
977. Mattke S, Hanson M. Expected wait times for access to a
disease-modifying Alzheimer’s treatment in the United States.
Alzheimer’s Dement. 2021;1-4.
978. Alzheimer’s Impact Movement. A path to better dementia care:
Alzheimer’s Impact Movement (AIM) and the Alzheimer’s
Association. Accessed January 30, 2024. Available at: https://
portal.alzimpact.org/media/serve/id/6077043a225cb.
979. Hirschman KB, Hodgson NA. Evidence-based interventions for
transitions in care for individuals living with dementia.
Gerontologist 2018;58(suppl_1):S129-S140.
980. Centers for Medicare & Medicaid Services. What is care
coordination? Care Coordination Measures Atlas Update.
Publication 14-0037-EF. Last reviewed June 2014. Accessed
January 30, 2024. Available at: https://www.ahrq.gov/ncepcr/
care/coordination/atlas/chapter2.html.
981. Centers for Medicare & Medicaid Services. Care coordination.
Accessed February 12, 2024. Available at: https://www.cms.gov/
priorities/innovation/key-concepts/care-coordination.
982. U.S. Department of Health and Human Services. National
Plan to Address Alzheimer’s Disease: 2023 Update.
Accessed February 12, 2024. Available at:
https://aspe.hhs.gov/sites/default/files/documents/
3c45034aec6cf63414b8ed7351ce7d95/ napa-national-
plan-2023-update.pdf.
983. Alzheimer’s Impact Movement (AIM). Building a path to better
dementia care. Accessed January 30, 2024. Available at: https://
alzimpact.org/Building-a-Path-to-Better-Dementia-Care.
984. Alzheimer’s Impact Movement. Comprehensive Care for
Alzheimer’s Act fact sheet. Alzheimer’s Impact Movement (AIM)
and the Alzheimer’s Association. March 2023. Accessed
January 30, 2024.Available at: https://alzimpact.org/sites/
default/files/2023-03/Comprehensive%20Care%20for%20
Alzheimer%27s%20Act%20%281%29.pdf.
985. Alzheimer’s Association. Medicare to improve dementia care for
individuals living with Alzheimer’s disease, caregivers [press
release]. July 31, 2023. Accessed January 30, 2024. Available
at: https://www.alz.org/news/2023/cms-dementia-care-
caregivers-initiative-guide.
986. Centers for Medicare & Medicaid Services. Guiding an Improved
Dementia Experience (GUIDE) Model. Accessed January 30,
2024. Available at: https://www.cms.gov/priorities/innovation/
innovation-models/guide.
987. Bernstein A, Harrison KL, Dulaney S, Merrilees J, Bowhay A,
Heunis J, et al. The role of care navigators working with people
with dementia and their caregivers. J Alzheimers Dis
2019;71(1):45-55.
988. Centers for Disease Control and Prevention. Patient
navigation. Last reviewed December 1, 2022. Accessed January
30, 2024. Available at: https://www.cdc.gov/cancer/community-
resources/interventions/patient-navigation.htm.
989. Chan RJ, Milch VE, Crawford-Williams F, Agbejule OA, Joseph
R, Johal J, et al. Patient navigation across the cancer care
continuum: An overview of systematic reviews and emerging
literature. CA Cancer J Clin 2023;73(6):565-89.
990. Kallmyer BA, Bass D, Baumgart M, Callahan CM, Dulaney S,
Evertson LC, et al. Dementia care navigation: Building toward a
common definition, key principles, and outcomes. Alzheimers
Dement (NY) 2023;9(3):e12408.
991. McBrien KA, Ivers N, Barnieh L, Bailey JJ, Lorenzetti DL,
Nicholas D, et al. Patient navigators for people with chronic
disease: A systematic review. PLoS One 2018;13(2):e0191980.
992. Ahuja R, McDermott M, Ty D, for the Milken Institute Alliance
to Improve Dementia Care. Guiding the care journey: Building
dementia workforce and system capacity through care
navigation. 2023. Accessed January 30, 2024. Available at:
https://milkeninstitute.org/sites/default/files/2023-03/
Guiding%20the%20Care%20Journey-%20Building%20
Dementia%20Workforce%20and%20System%20Capacity%20
through%20Care%20Navigation.pdf.
993. Alzheimer’s Association. Dementia care navigation guiding
principles. Accessed January 30, 2024. Available at: https://
www.alz.org/professionals/health-systems-medical-
professionals/dementia-care-guiding-principles
994. Fazio S, Pace D, Maslow K, Zimmerman S, Kallmyer B.
Alzheimer's Association Dementia Care Practice
Recommendations. Gerontologist 2018;58(suppl_1):S1-S9.
995. Patient navigator. National Cancer Institute (NCI) Dictionary of
Cancer Terms. Accessed January 30, 2024. Available at: https://
www.cancer.gov/publications/dictionaries/cancer-terms/def/
patient-navigator.
996. Health Resources & Services Administration (HRSA) Health
Workforce. Train health care workers about dementia.
Reviewed January 2023. Accessed February 2, 2024. Available
at: https://bhw.hrsa.gov/alzheimers-dementia-training.
997. Alzheimer's Association. Recognized dementia care training
programs. Accessed February 2, 2024. Available at: https://
www.alz.org/professionals/professional-providers/dementia-
care-training-certification/recognized-dementia-care-
training-programs.
145
998. UCSF Memory and Aging Center. Care ecosystem toolkit.
February 2018. Accessed February 2, 2024.
Available at: https://bettercareplaybook.org/resources/
care-ecosystem-toolkit.
999. Wisconsin Alzheimer’s Institute. Training home health
clinicians to support caregivers. Accessed February 2, 2024.
Available at: https://wai.wisc.edu/dementia-capable-
wisconsin/home-health-caregiver-education/.
1000. UCLA Health. Dementia care caregiver training videos.
Accessed February 2, 2024. Available at: https://www.
uclahealth.org/medical-services/geriatrics/dementia/
caregiver-education/caregiver-training-videos.
1001. National Alzheimer’s and Dementia Resource Center
website. Accessed February 2, 2024. Available at: https://
nadrc.acl.gov/home.
1002. National Association of Community Health Centers. Brain
health. Accessed February 2, 2024. Available at: https://www.
nachc.org/topic/brain-health/.
1003. Healthcare.gov. Fee for service. Accessed February 2, 2024.
Available at: https://www.healthcare.gov/glossary/fee-for-
service/#:~:text=A%20method%20in%20which%20
doctors,include%20tests%20and%20office%20visits.
1004. Guterman S. Wielding the carrot and the stick: how to move
the U.S. health care system away from fee-for-service
payment. The Commonwealth Fund blog. August 27, 2013.
Accessed February 2, 2024. Available at: https://www.
commonwealthfund.org/blog/2013/wielding-carrot-
and-stick-how-move-us-health-care-system-away-fee-
service-payment.
1005. Werner RM, Emanuel EJ, Pham HH, Navathe AS. The future
of value-based payment: a road map to 2030. University of
Pennsylvania Leonard Davis Institute of Health Economics.
February 17, 2021. Accessed February 2, 2024. Available at:
https://ldi.upenn.edu/our-work/research-updates/the-
future-of-value-based-payment-a-road-map-to-2030/.
1006. Alzheimer’s Impact Movement. Dementia care management:
a proposed framework for an alternative payment model.
Alzheimer’s Impact Movement (AIM) and the Alzheimer’s
Association. Accessed February 2, 2024. Available at: https://
portal.alzimpact.org/media/serve/id/5f1b511b98110.
1007. Baumgart M. An alternative payment model could deliver
better care to people with dementia - and save Medicare
money. Alzheimer’s Impact Movement (AIM) blog. Accessed
February 23, 2024. Available at: https://portal.alzimpact.org/
media/serve/id/5f1b511b98110.
1008. Alzheimer’s Association. Helpline. Accessed February 2, 2024.
Available at: https://www.alz.org/help-support/resources/helpline.
1009. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s
disease in the United States and the public health impact of
delaying disease onset. Am J Public Health 1998;88:1337-42.
1010. U.S. Department of Labor. Changes in Basic Minimum Wages in
Non-Farm Employment Under State Law: Selected Years 1968
to 2020. Available at: https://www.dol.gov/agencies/whd/state/
minimum-wage/history. Accessed December 15, 2023.
1011. U.S. Centers for Medicare & Medicaid Services. Your coverage
options. Available at: https://www.medicare.gov/basics/
get-started-with-medicare/get-more-coverage/your-
coverage-options. Accessed December 11, 2023.

146 Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2024;20(5).
The Alzheimer’s Association acknowledges the
contributions of Joseph Gaugler, Ph.D., Bryan
James, Ph.D., Tricia Johnson, Ph.D., Jessica Reimer,
Ph.D., Kezia Scales, Ph.D., Sarah Tom, Ph.D., M.P.H.,
Jennifer Weuve, M.P.H., Sc.D., and Jarmin Yeh,
Ph.D., M.P.H., M.S.S.W., in the preparation of
2024 Alzheimer’s Disease Facts and Figures.

Alzheimer’s Association
225 N. Michigan Ave., Fl. 17
Chicago, IL 60601-7633
800.272.3900
alz.org
®
©2024 Alzheimer’s Association. All rights reserved.
This is an official publication of the Alzheimer’s Association
but may be distributed freely and without charge by
unaffiliated organizations and individuals. Such distribution
does not constitute an endorsement of these parties or
their activities by the Alzheimer’s Association.
The Alzheimer’s Association leads the way to
end Alzheimer’s and all other dementia —
by accelerating global research, driving risk
reduction and early detection, and maximizing
quality care and support.
Our vision is a world without Alzheimer’s and
all other dementia.®
