
Diabetes treatment algorithms
Note: the treatment algorithms are updated routinely. The most recent versions will appear online.
Please be sure to use the most recent version by accessing the Texas Diabetes Council Web site at
www.texasdiabetescouncil.org
A1c Goals
Texas Diabetes Council A1c Goals – Approved: 10/29/09
Diabetes Minimum Practice Recommendations – revised: 10/29/09
Prevention
Prevention and Delay of Type 2 Diabetes in Children and Adults with Impaired Fasting Glucose
(IFG) and/or Impaired Glucose Tolerance (IGT) –
revised: 01/27/05
Weight Loss
Weight Loss Algorithm for Overweight and Obese Adults – revised: 01/27/05
Weight Management Algorithm for Overweight Children and Adolescents – Approved: 04/28/05
Exercise
Exercise Algorithm Type 2 Diabetes Prevention and Therapy – revised: 01/22/04
Nutrition
Diabetes Medical Nutrition Therapy and Prevention Algorithm for Adults – revised: 07/22/10
Nutrition Recommendations and Interventions for Diabetes (supplement) – Approved: 10/29/09
Glycemic Control
Glycemic Control Algorithm for Type 2 Diabetes Mellitus in Adults – revised: 07/22/10
Cardiovascular Risk
Reduction
Hypertension Algorithm for Diabetes in Adults – revised: 01/26/12
Lipid Algorithm for Type 1 and Type 2 Diabetes Mellitus in Adults – revised: 01/24/08
Macrovascular Risk Reduction in Diabetes: Antiplatelet Therapy (supplement) – publicAtion dAte: 2004
Insulin Administration
Insulin Algorithm for Type 1 Diabetes Mellitus in Children and Adults – revised: 01/27/10
Insulin Algorithm for Type 2 Diabetes Mellitus in Children and Adults – revised: 10/28/10
Initiation of Once Daily Insulin Therapy for Type 2 Diabetes Mellitus in
Children and Adults –
revised: 10/28/10
Worksheet: Advancing to Intensive/Physiologic Basal: Bolus Insulin Therapy – revised 01/27/10
IV Insulin Infusion Protocol for Critically-Ill Adult Patients in the ICU Setting – revised: 10/25/07
ICU Insulin Orders – I.V. Insulin Infusion Protocol – revised: 02/21/08
Orders for Adults with DKA and Hyperglycemic Hyperosmolar State (HHS) – Approved: 07/31/08
Transition Algorithm from I.V. to S.Q. Insulin for Patients with Diabetes or Hyperglycemia – Approved: 07/31/08
Transition from I.V. to S.Q. Insulin Order Set Eating Status NPO or PO – Approved 10/27/11
Transition from I.V. to S.Q. Insulin Order Set TPN or Enteral (Tube) Nutrition – Approved 10/27/11
Insulin Pump Therapy (supplement)
Foot Care
Diabetic Foot Care – Approved: 04/23/04
Diabetic Foot Screen – Approved: 04/23/04
Diabetic Foot Exam – Approved: 04/23/04
Diabetic Foot Care/Referral Algorithm – Approved: 04/23/04
High Risk Scenario and Ulcer Management – Approved: 04/23/04
Foot Screening Mapping Examples (supplement)
Pain Management
Recommendations for Treatment of Painful Peripheral Diabetic Neuropathy in Adults – Approved: 04/26/07
Care of the Elderly
Considerations for Elderly Persons with Diabetes (supplemen t)
Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities (suppleme nt)
Screening and Management of Hyperglycemia in the Geriatric Population –
Approved: 10/23/08
Authors
Texas Diabetes Council Authorship – Minimum Practice Recommendations,
Algorithms and Reports –
revised: 12/04/08
Treatment Algorithms, Protocols, Guidelines,
and Recommendations

References
1. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2
diabetes. N Engl J Med 2008;358:2545-2559.
2. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2
diabetes. N Engl J Med 2008;358:2560-2572.
3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on
the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med
1993;329:977-986.
4. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/
EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1
diabetes. N Engl J Med 2005;353:2643-2653.
5. Gæ
de P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular
disease in patients with type 2 diabetes. N Engl J Med 2003;348:383-393.
6. Gæ
de P, Lund-Anderson H, Parving H-H, Pedersen O. Effect of a Multifactorial Intervention on Mortality in Type
2 Diabetes. N Engl J Med 2008;358:580-591.
7. Ho
lman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type
2 diabetes. N Engl J Med 2008;359:1577-1589.
8. Oh
kubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular
complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year
study. Diabetes Res Clin Pract 1995;28:103-117.
9. Re
ichard P, Bengt-Yngve N, Rosenqvist U. The effect of long-term intensified insulin treatment on the development
of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304-309.
1
0.
Sh
ichiri M, Ohkubo Y, Kishikawa H, Wake N. Long term results of the Kumamoto Study on optimal diabetes
control in type 2 diabetic patients. Diabetes Care 2000;23:Suppl 2:B21-B29.
11
.
UK
Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin
compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet
1998;352:837-853.
12
.
UK
Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on
complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-865.
13
.
Th
e Veterans Affairs Diabetes Trial Investigators. Glucose Control and Vascular Complications in Veterans with
Type 2 Diabetes. N Engl J Med 2009;360:129-139.
Texas Diabetes Council
A1c Goals
A1c is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA Clinical Practice Recommendations.
Diabetes Care 2009;32(suppl 1):S19-20
A1c Goals
Individualize goal based on patient risk factors
A1c < 6-7% A1c < 7-8%
Intensify management if:
• Absent/stable
cardi
ovascular
dise
ase
• Mild-moderate
micr
ovascular
compl
ications
• Intact
hypo
glycemia
awar
eness
• Infrequent
hypo
glycemic
epis
odes
• Recently
diag
nosed
diab
etes
Less intensive management if:
• Evidence
ofadva
nced
orpoorl
y
contr
olled
cardi
ovascular
and/o
r
micr
ovascular
comp
lications
• Hypoglycemia
unaw
areness
• Vulnerablepatient(ie,impairedcognition,
dementia,fallhistory)
Diabetes treatment algorithms
1 of 1 – A1c Goals – Approved 10/29/09
Approved 10/29/09
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
Diabetes Minimum Practice
Recommendations
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp1 of 1 – Diabetes Minimum Practice Recommendations – Revised 10/29/09
Publication # 45-12085 Revised 10/29/09
1. Complete history & physical
Ini
tial visit and at clinician’s discretion
(including risk factors, exercise & diet)
Dat
e
Re
sult
2.
Di
abetes Education
1
Initial visit and at clinician’s discretion
Dat
e
Re
sult
3.
Med
ical Nutrition Therapy
Ini
tial visit and at clinician’s discretion
Dat
e
Re
sult
4.
Ex
ercise Counseling
Ini
tial visit and at clinician’s discretion
Date
Result
5.
Ps
ychosocial Counseling
Ini
tial visit and at clinician’s discretion
Dat
e
Re
sult
6.
Lif
estyle/Behavior Changes Counseling
Initial visit and at
clinician’s discretion
Sm
oking cessation
Date
Result
Alcohol reduction
7. Weight/Height/BMI
Adult Overweight=BMI 25–29.9
Adult Obesity=BMI ≥ 30
Every Visit
Date
Result
8.
Bl
ood Pressure
Tar
get: <130/80 mm Hg
Tar
get: < 125/75 mm Hg if ≥ 1g proteinuria
Ever
y
Visit
Dat
e
Re
sult
9. Foot Inspection
Visual inspection for skin and nail lesions, calluses,
infections
Every Visit
Dat
e
Re
sult
10.
Or
al/Dental Inspection
Ref
er for dental care annually or as needed
Ever
y
Visit
Date
Result
11.
Gr
owth and Development
(including height) in
Chil
dren
Ever
y
Visit
Dat
e
Re
sult
12. Aspirin/Antiplatelet Prophylaxis
(if no contraindications) Type 1 or 2 ≥ age 30
Every Visit
Dat
e
Re
sult
13.
A
1c2
Ind
ividualize goal based on patient risk factors
Int
ensive management - A1c < 6-7%
Les
s intensive management – A1c <7-8%
Every 3–6 months
Date
Result
14.
Ki
dney evaluation
Est
imate GF
R (
eGF
R) & mi
croalbumin determination
(>30mg = abnormal).
Con
sider nephro/endocrine evaluation at
Sta
ge 3
CKD (
eGF
R <
60); also consider
P
T
H &
H
gb if
CKD Sta
ge 3
If s
ignificant proteinuria; monitor serum creatinine
every 3–6 months
Typ
e 1: Annually beginning 5 years from
diagnosis
Typ
e 2:
Ini
tial visit then annually
Dat
e
Re
sult
15.
Dilated funduscopic eye exam
By an ophthalmologist or therapeutic
optometrist
Type I: Annually beginning 5 years from
diagnosis
Type 2: Initial, then annually
Date
Result
16. Or
al/Dental Exam
Ref
er to appropriate provider
Annually or as needed
Dat
e
Re
sult
17.
Foot Exam
Complete foot exam and neurologic assessment
Annually or as needed
18. Li
pid Profile
Tar
gets:
L
DL
-C <
100 mg/d
L (CHD <
70mg/d
L)
Tri
glycerides <150 mg/d
L
An
nually if at goal; otherwise every 3–6
months (> age 18)
Dat
e
Re
sult
19.
Immunizations
Influenza (Flu) Vaccine
Td Vaccine
Pneumococcal Vaccine
Childhood Immunizations
Annually
Every 10 Years
Initial; repeat per ACIP
Per CDC Schedule
Date
Result
1
Diabetes Education should address the following: self-management skills (i.e. monitoring, sick day management), medications, frequency of hypoglycemia, high-risk behaviors
(e.g. smoking, alcohol), adherence with self-care (self-management plan from the last visit including diet, medication use, exercise plan), assessment of complications, diabetes
knowledge and follow-up of referrals.
2
Intensify management if: Absent/stable cardiovascular disease, mild-moderate microvascular complications, intact hypoglycemia awareness, infrequent hypoglycemic episodes,
recently diagnosed diabetes. Less intensive management if: Evidence of advanced or poorly controlled cardiovascular and/or microvascular complications, hypoglycemia
unawareness, vulnerable patient (ie, impaired cognition, dementia, fall history).
Name: ID#: D.O.B.: Sex: M F
Exam/Test/Counseling Schedule
Suggested Result Codes: O=Ordered, N=Normal, A=Abnormal, E=Done Elsewhere, R=Referred

Prevention and Delay of Type 2 Diabetes in Children and Adults with
Impaired Fasting Glucose (IFG) and/or Impaired Glucose Tolerance (IGT)
1 of 2 – Prevention and Delay of Type 2 Diabetes in Children and Adults with Impaired Fasting Glucose (IFG) and/or Impaired Glucose Tolerance (IGT) – Revised 01/27/05
Screening
1
:
1. General population; BMI ≥25
Ind
ividuals ≥45 years
Bas
eline and q 3 years
2.
Hi
gh risk population ≥18 years;
BMI ≥25
Bas
eline and yearly
3.
Ch
ildren and youth at risk
Bas
eline at age 10 and q 2 years
· Overweight BMI (≥85
th
%’ile for age and gender and ≥ two
risk factors)
Risk Factors:
· 1
st
degree (and/or 2
nd
degree in children) relative with diabetes
·
Hx o
f gestational diabetes or delivery of a baby weighing
>9 lbs
· High-risk ethnic group
·
H
ypertension
· Dyslipidemia
·
P
ol
ycystic
Ova
ry
Syn
drome
·
M
etabolic
2
and/or Insulin Resistance
3
Syndromes
·
Vas
cular disease
·
Ac
anthosis nigricans
NORMAL
F
PG <1
00 mg/d
L;
2
-hr
OGTT <
140 mg/d
L
BMI Body mass index (kg/m
2
)
FPG
Fa
sting plasma glucose
OGTT
1.
75g/kg to max 75g
Ora
l glucose tolerance test
PCP
Pri
mary care provider
TESTING
1,4,5
: FPG Recommended: (if abnormal confirm X 1); however
2-hr
OGTT a
cceptable in adults and should be used for diagnosis in
children
6
(routine measurement of insulin levels is not recommended)
Diagnosis:
IFG
FPG ≥100 and <126 mg/dL
and/or
IGT 2-hr OGTT
Gl
ucose ≥140 and <200 mg/d
L
Diagnosis: Type 2
Diabetes
FPG ≥126 mg/dL and/or 2-hr
OGTT ≥
200 mg/d
L—
Ref
er to
Tex
as
Dia
betes
Cou
ncil
Algorithms
Rescreen Based on
Risk
Factors
Continue Aggressive
Management of
Modifiable
Ris
k Factors
and Appropriate
Life
style
Inte
rvention
Unsuccessful Outcome: Children
Abnormal 2-hr
OGTT—Int
ervention and
Con
tinue
Lif
estyle
Ref
er to
Ped
iatric
End
ocrinologist or
Obe
sity
Spe
cialist
Initial Intervention: Lifestyle
7,8
Weight Loss: 5–10% if BMI ≤40; 10–15% if BMI >40
Exercise/Physical Activity: ≥30–60 minutes per day
Hypocaloric diet:
Def
icit 250–1000 Kcal per day ± Meal
Replacements
Behavior Modification:
Nut
rition/Family
Cou
nseling
Reg
ular Follow-up by
P
CP
Unsuccessful Outcome: Adults
Abnormal F
PG an
d/or 2-hr
OGTT—Con
sider Adding
Dru
g
Thera
py
9
to Lifestyle Intervention
Metformin
8,10
Contraindicated
in
Renal
Dise
ase,
Liv
er
Dis
ease,
CHF
Orlistat
11
Con
traindicated
in
Chr
onic
Malabsorption,
Cho
lestasis
Acarbose
12
Contraindicated
in Gastrointestinal
Dise
ase
Reassess FPG and/or 2-hr OGTT every 6 months
Abnormal—
Re-
evaluate
Lif
estyle and Medication
Reg
imen
Normal—
Con
tinue
Cur
rent
Thera
py
Successful Outcome
Nor
mal F
PG an
d/or 2-hr
OGTT Lif
estyle
Maintenance—
Continue Physical Activity and Weight
Los
s/Maintenance
Reassess yearly
F
PG an
d/or 2-hr
OGTT
Abnormal—
Con
sider
Dru
g
Thera
py
9
Normal—
Conti
nue
Life
style
Inte
rvention
6 months
6 months
Revised 01/27/05Publication #45-11825
Diabetes treatment algorithms
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Footnotes:
1. American Diabetes Association: Clinical Practice Guidelines 2004. Screening for type 2 diabetes. Diabetes Care. 2004;27(suppl 1):S11-4; Diabetes Care.
2005;28(suppl 1):S4-S36.
2. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).
JAMA. 2001;285(19):2486–97.
3. Am
erican College of Endocrinology position statement on the insulin resistance syndrome.
Endocr Pract. 2003;9(3):237-52.
4. Am
erican Diabetes Association: Clinical Practice Guidelines 2004. The prevention or delay of type 2 diabetes. Diabetes Care. 2004;27(suppl 1):S47-54; Diabetes
Care. 2005;28(suppl 1):S4-S36.
5. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes.
1997;46(4):701-10.
6. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802-
10. Erratum in: N Engl J Med. 2002;346(22):1756. Correction of dosage error in abstract.
7. See Texas Diabetes Council algorithms for treatment of exercise, weight loss, and nutrition.
8. Kn
owler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle
intervention or metformin. N Engl J Med. 2002;346(6):393-403 (dose of metformin 850 mg twice daily).
9. No medication is currently FDA-approved for prevention of type 2 diabetes in adults, but a number of studies provide evidence for drug treatment.
10. Metformin is as effective as lifestyle intervention in individuals <age 45 or those with BMI ≥35; metformin is nearly ineffective in individuals ≥age 60 or those with
BMI <30 (DPP evidence).
11. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an
adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155-61 (dose of orlistat 120 mg three times daily with
food).
12. Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072-7
(dose of acarbose 100 mg three times daily with food).
2 of 2 – Prevention and Delay of Type 2 Diabetes in Children and Adults with Impaired Fasting Glucose (IFG) and/or Impaired Glucose Tolerance (IGT) – Revised 01/27/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Pt Motivated and Adherent
Class 3 Obesity
2
BMI ≥
40
Pt Motivated
Tar
gets
Not
Maintained
Tar
gets Maintained
Tar
gets
Not M
et
Pt Not
Motivated
3–6 months
Consider Obesity Pharmacologic Monotherapy as Adjunct to
Lif
estyle
Cha
nges if:
BMI ≥
27 with
Com
orbidities or if
BMI ≥
30
Pt Not Motivated
6 months
Targe
ts
Not Met
BMI = Wt in kg
(H
t in m)
2
= Wt in lb x 703
(H
t in inches)
2
Normal
BMI 18.
5 –24.9
Obtain Accurate Height (Ht) and Weight (Wt);
Calculate Body Mass Index (BMI) (
See Tab
le on
Pag
e 2 of 2)
Wt Loss
Maintenance
Offer Medically-Supervised
Wt Loss Intervention
8
Targets Met
Assess Comorbidities and Risk Factors
3
Metabolic
3
or Insulin Resistance
4
Syndromes; Waist Circumference (Men >40 inches; Women >35 inches);
Dyslipidemia
3
(Elevated LDL-C and/or TG; Low HDL-C); HTN
5
;
Imp
aired Fasting Glucose (F
PG 10
0-125 mg/d
L); Imp
aired Glucose
Tol
erance (
Pos
t-challenge
PG 14
0 –199 mg/d
L);
Diabetes Mellitus
6
(FPG ≥126 mg/dL; Post-challenge PG ≥200 mg/dL);
Coronary Heart or Other Vascular Disease (CVD); Sleep Apnea; DJD; GERD; Gallstones; NAFLD/NASH; Polycystic Ovary Syndrome; Urinary
Inc
ontinence
Wt LossTargets
2-3 unit Reduction in BMI
≥5-10% Reduction in Wt
(≥15% Wt Loss for Class 3 Obese Pts)
+
Si
gnificant Improvement in Comorbidities
Maintain Healthy Lifestyle, Diet, and
Exe
rcise and Monitor Wt Weekly for
Lif
e with
Per
iodic Follow-up by H
CP
Consider Referral for Bariatric
Sur
gery
14
as Adjunct to Lifestyle
Cha
nges if
BMI ≥
35 with
Com
orbidities or if
BMI ≥
40
Education;
Lifestyle Change–Hypocaloric Diet
(Deficit 250–1000 Kcal/d
9
± Meal Replacements);
Exercise (≥30-60 minutes/day);
Behavior Modification;
Nut
rition/Family
Cou
nseling
If Unsuccessful in 4 –12 Weeks
(with Motivated/Adherent
Pt),
Consider Switching Drug Class or
Using Combina
tion
Thera
py
13
Overweight
BMI 25
–29.9
Obesity
2
BMI ≥
30
Consider Contributing Factors
Dru
gs
7
Hypothyroidism
Cus
hing
Syn
drome
Male Hypogonadism
Adult GH
Defi
ciency
Educate; Manage Risk Factors
Aggressively, and Reassess
Rea
diness to
Change
Peri
odically
Appetite Suppressants
Phentermine
10
Contraindicated in: Uncontrolled
H
TN; C
VD
; Ar
rhythmia;
Str
oke;
CHF
; Hx;
Sub
stance Abuse;
Glaucoma (
Narr
ow-angle);
Con
current MA
OI Rx
Sibutramine
11
Contraindicated in:
Unco
ntrolled H
TN;
Gl
aucoma (
Nar
row-angle);
Arrhythmia;
Stroke; CVD;
CHF
;
Con
current
S
SRI
& M
A
OI
Thera
py
Lipase Inhibitor Orlistat
11,12
Supported by Evidence in
Type
2
Dia
betes Mellitus;
Con
traindicated in:
Chr
onic
Malabsorption;
Cho
lestasis;
Orl
istat Hypersensitivity;
Con
current
Cycl
osporin
The
rapy
Reinforce Healthy Lifestyle, Diet,
and
Exe
rcise
Targets Not Met
Consider Obesity Pharmacotherapy
for Maintenance of Wt
Los
s
(
See
Phar
macotherapy
Box
)
Weight Loss Algorithm for
Overweight and Obese Adults
1
Revised 01/27/05Stock # 45-11694
1 of 2 – Weight Loss Algorithm for Overweight and Obese Adults – Revised 01/27/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Body Mass Index Table
Abbreviations
CHF
Congestive Heart Failure
CVD
Cardiovascular Disease
DJD
Degenerative Joint Disease
FPG
Fasting Plasma Glucose
GERD
Gastro-esophageal Reflux Disease
HCP
Health Care Professional
HDL-C
High-density Lipoprotein Cholesterol
HTN
Hypertension
LDL-C
Low-density Lipoprotein Cholesterol
MAOI
Monoamine Oxidase Inhibitors
NAFLD
Non-alcoholic Fatty Liver Disease
NASH
Non-alcoholic Steatohepatitis
SSRI
Selective Serotonin Reuptake Inhibitors
TG
Triglycerides
Footnotes:
1
Adapted from NIH/NHLBI/NAASO;1998; NIH Publication No. 98-4083 (Obes Res 1998; 6[Suppl 2]:51S-210S)
2
Consider starting obesity pharmacotherapy concurrent with other treatment modalities at presentation in motivated/adherent pts if BMI ≥35 with comorbidities or
≥40 with no comorbidities
3
National Cholesterol Education Program-Adult Treatment Panel III. JAMA 2001; 285:2466-2497
4
American Association of Clinical Endocrinologists Consensus Conference on the Insulin Resistance Syndrome, Washington, DC; August 2002 (Diabetes Care 2003; 26:1297-1303)
5
The 7th Report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC 7). JAMA 2003; 289: 2560-2572
6
See Glycemic Control Algorithm in Type 2 Diabetes Mellitus in Children and Adults; Diabetes medications may need to be adjusted to avoid hypoglycemia in pts who lose wt
7
Most antipsychotics, tricyclic antidepressants, lithium, valproic acid, carbamazepine, insulin/insulin analogs, sulfonylureas, thiazolidinediones, cyproheptidine,
glucocorticoids, and estrogens/progestins may be associated with wt gain
8
Assuming BMI ≥25 and/or waist circumference >40 inches in men, >35 inches in women, and one or more major comorbidity
9
Calorie deficit of 250 Kcal/day will result in ~1/2 lb/week wt loss (1000 Kcal/day ~2 lb/week wt loss)
10
FDA-approved for adjunctive short-term use ≤3 months for wt loss; see drug prescribing brochure; ~Cost–$0.85/30 mg pill (generic- AWP 2003)
11
FDA-approved for use for up to 2 years for wt loss and maintenance of wt loss; see drug prescribing brochures; ~Cost– sibutramine $3.64/15 mg pill; orlistat
$1.38/120 mg pill (AWP 2003)
12
Diabetes Care 1998; 21:1288-1294; Diabetes Care 2002; 25:1033-1041; Diabetes Care 2002; 25:1123-1128
13
Orlistat can be combined with the other agents; sibutramine and phentermine are not to be used in combination
14
After minimum of 6 months of intensive wt loss management (including obesity pharmacotherapy if no contraindications) in motivated and adherent pts
120
30
29
28
27
26
25
24
23
23
22
21
21
20
19
19
18
18
17
17
16
16
15
15
15
14
14
14
13
13
13
130140 150160 170180
33 35 38 40 43 45
31 34 36 39 41 43
30 33 35 37 40 42
29 31 34 36 38 40
28 30 33 35 37 39
27 29 31 34 36 38
26 28 30 32 34 36
25 27 29 31 33 35
25 27 28 30 32 34
24 26 27 29 31 33
23 25 27 28 30 32
22 24 26 28 29 31
22 23 25 27 28 30
21 23 24 26 27 29
20 22 24 25 27 28
20 21 23 24 26 27
19 21 22 24 25 27
19 20 22 23 24 26
18 20 21 22 24 25
18 19 20 22 23 24
17 19 20 21 22 24
17 18 19 21 22 23
16 18 19 20 21 23
16 17 18 20 21 22
15 17 18 19 20 21
15 16 17 19 20 21
15 16 17 18 19 20
14 15 17 18 19 20
14 15 16 17 18 19
14 15 16 17 18 19
4'5"
4'6"
4'7"
4'8"
4'9"
4'10"
4'11"
5'0"
5'1"
5'2"
5'3"
5'4"
5'5"
5'6"
5'7"
5'8"
5'9"
5'10"
5'11"
6'0"
6'1"
6'2"
6'3"
6'4"
6'5"
6'6"
6'7"
6'8"
6'9"
6'10"
190 200210 220230 240250
48505355586063
46 48 51 53 56 58 60
44 47 49 51 54 56 58
43454749525456
41434648505254
40424446485052
38404345474951
37394143454749
36384042444547
35373840424446
34363739414344
33343638404143
32333537384042
31323436373940
30313335363839
29303234353738
2830313
3343637
27293032333536
27282931323435
26272930313334
25262829303233
24262728303132
24252628293031
23242627282930
23242526272930
22232425272829
21232425262728
21222324252628
20212324252627
20212223242526
260270 280290 300
65 68 70 73 75
63 65 68 70 72
61 63 65 68 70
58 61 63 65 67
56 59 61 63 65
54 57 59 61 63
53 55 57 59 61
51 53 55 57 59
49 51 53 55 57
48 49 51 53 55
46 48 50 51 53
45 46 48 50 52
43 45 47 48 50
42 44 45 47 49
41 42 44 46 47
40 41 43 44 46
38 40 41 43 44
37 39 40 42 43
36 38 39 41 42
35 37 38 39 41
34 36 37 38 40
33 35 36 37 39
33 34 35 36 38
32 33 34 35 37
31 32 33 34 36
30 31 32 34 35
29 30 32 33 34
29 30 31 32 33
28 29 30 31 32
27 28 29 30 31
310320 330
78 80 83
75 77 80
72 75 77
70 72 74
67 69 72
65 67 69
63 65 67
61 63 65
59 61 62
57 59 60
55 57 59
53 55 57
52 53 55
50 52 53
49 50 52
47 49 50
46 47 49
45 46 47
43 45 46
42 43 45
41 42 44
40 41 42
39 40 41
38 39 40
37 38 39
36 37 38
35 36 37
34 35 36
33 34 35
32 34 35
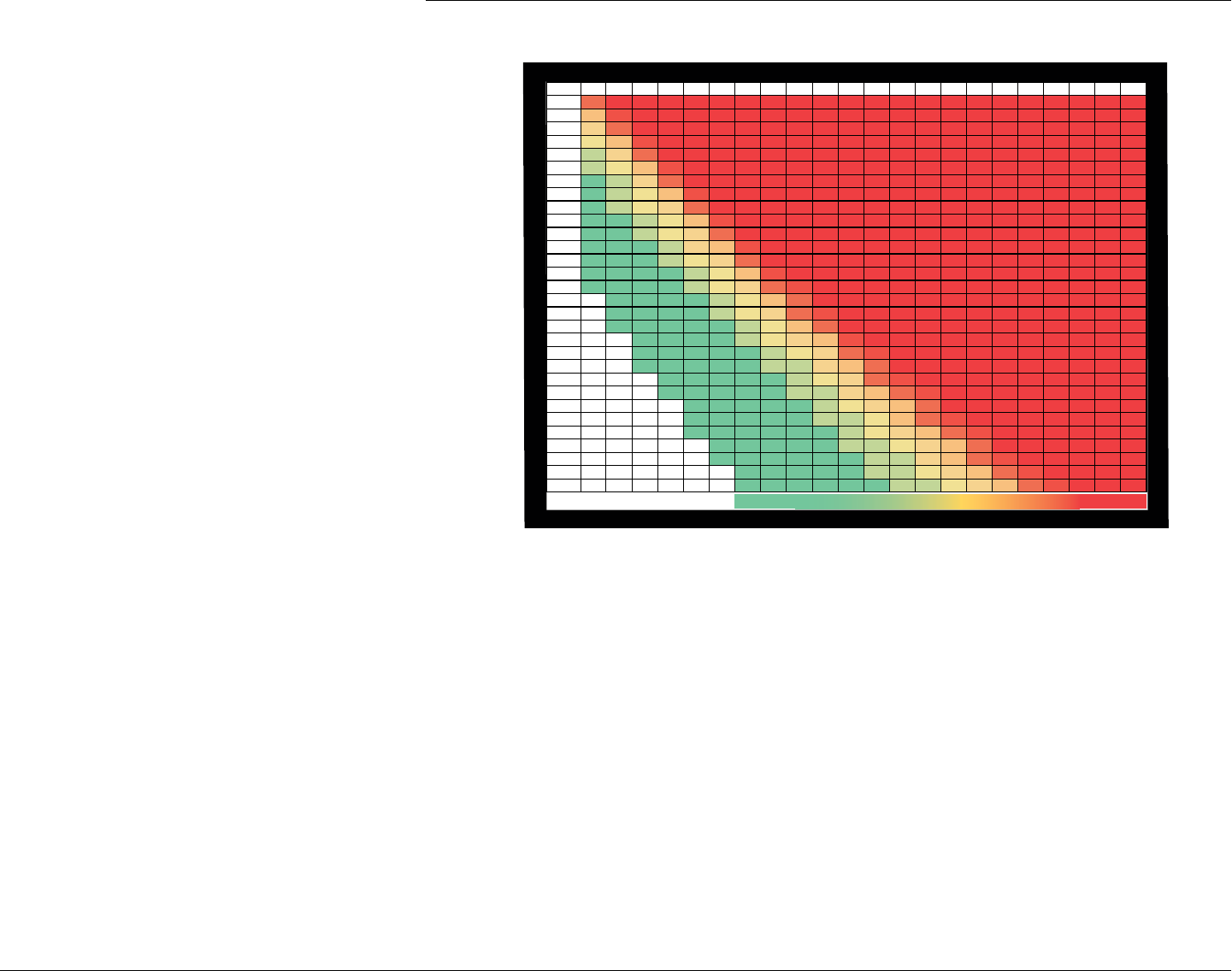
WEIGHT (lb)BMI TABLE
HEIGHT (ft/in)
ksir eroMksir sseL
2 of 2 – Weight Loss Algorithm for Overweight and Obese Adults – Revised 01/27/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Weight Management Algorithm for
Overweight Children and Adolescents
1
Pt/Family Motivated
Pt/Family Not Motivated
Targets Met
Educate Patient and Family; Manage
Comorbidities and Risk Factors;
Rea
ssess
Rea
diness
Per
iodically
Consider Obesity Pharmacologic Monotherapy as Adjunct to
Lifestyle Changes if BMI ≥27 with Comorbidities or if BMI ≥30
Appetite Suppressant
Sibutramine
6
Contraindicated in: Uncontrolled
HTN; Glaucoma (Narrow-angle);
Arrhythmia;
C
V
A; C
VD
; CHF
;
Con
current
S
SRI
; MAOI The
rapy;
Preg
nancy;
Bul
imia/Anorexia
Ner
vosa Hx
Lipase Inhibitor Orlistat
7
Contraindicated in:
Chronic Malabsorption;
Cho
lestasis;
Orl
istat
Hypersensitivity;
Con
current
Cycl
osporin
The
rapy;
Pre
gnancy
Targets Not Met
Motivated & Targets Not Met
Wt Loss
Maintenance
Obtain Accurate Height (Ht) & Weight (Wt)
Calculate Body Mass Index (BMI-see page 3 of 4)
(Refer to gender-specific BMI Chart on page 4 of 4)
At Risk for Overweight
2
85%–95th%ile
BMI f
or Age & Gender;
Rap
id Wt Gain
2
Overweight
2
>95th%ile BMI for Age & Gender
Normal
2
<85
th
%ile BMI for Age & Gender
Reinforce Healthy Lifestyle, Diet, and Exercise;
Watch for Rapid Wt Gain
2
Assess for Comorbidities
3
Sleep Apnea; Pseudotumor Cerebri; Dyslipidemia (Elevated LDL-C and/or TG; Low HDL-C); HTN;
NAFLD; GERD; Wt-bearing Joint Pain; PCOS /Hyperandrogenism; AN; Psychological Adjustment Disorders; T2DM (FPG≥126 mg/dL;
Pos
t
Cha
llenge
PG ≥2
00 mg/d
L)
Assess for Risk Factors and/or Contributing Factors
Medications
4
; Hypothyroidism; Cushing Syndrome; Prader-Willi Syndrome; SGA; Low Birth Wt; Post Malignancy Treatment
Pt/Family Motivated
Pt/
Family Motivated
Initial Wt Loss Targets
5
(1st 6 Months)
u
>95th%ile BMI for Age & Gender
u
>85th%ile BMI with Comorbidities
Pubertal: = 10% Body Wt Loss
Pre-pubertal:
≥a
ge 7 = 1–2 lbs per Month Wt
Los
s
<age 7 = Wt Maintenance or
Modest Wt
Los
s
Deg
ree of Wt
Los
s will
Dep
end on the
Sev
erity of
the
Comor
bidity
Offer Medically Supervised Wt
Management Intervention
Education; Lifestyle Changes
2
; Nutrition;
Increased Physical Activity; Behavior
Modification; Nutrition/Family Counseling.
Pt/Family Not Motivated
≥6 months
Maintain Healthy Lifestyle, Diet and Exercise;
Reinforce Education; Monitor Wt Weekly for Life with
Per
iodic Follow-up by H
CP
Consider Bariatric Surgery
8
for adolescents
who meet criteria. (See page 2)
Targets Maintained
BMI = Wt in kg
(Ht in m)
2
= Wt in lb x 703
(H
t in inches)
2
≥6 months
≥6 months
Approved 04/28/05Publication # 45-12083
1 of 4 – Weight Management Algorithm for Overweight Children and Adolescents – Approved 04/28/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Footnotes:
1. Adapted from the Texas Council’s Weight Loss Algorithm for Overweight and Obese Adults
2. Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal
and Child Health Bureau, Health Resources and Services Administration and the Department of Health and
Human Services. Pediatrics. 1998;102(3):E29
3. Ba
rlow SE, Dietz WH. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal
and Child Health Bureau, Health Resources and Services Administration and the Department of Health and
Human Services. Pediatrics. 1998;102(3):E29; and American Diabetes Association. Type 2 diabetes in children
and adolescents. Pediatrics. 2000;105(3 Pt 1):671-80; Refer to appropriate Texas Diabetes Council algorithms
4. Medications that affect insulin sensitivity:
5. No evidence-based outcomes data are yet available for weight loss targets
6. Berkowitz RI, Wadden TA, Tershakovec AM, et al. Behavior therapy and sibutramine for the treatment of
adolescent obesity: a randomized controlled trial. JAMA. 2003;289(14):1805-12; sibutramine is FDA-approved
for ages ≥16 yr
7. McDuffie JR, Calis KA, Uwaifo GI, et al. Efficacy of orlistat as an adjunct to behavioral treatment in
overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr
Endocrinol Metab. 2004;17(3):307-19; orlistat is FDA-approved for ages ≥12 yr
8. In
ge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and
recommendations. Pediatrics. 2004;114(1):217-23
9. Rosner B, Prineas R, Loggie J, et al. Percentiles for body mass index in U.S. children 5 to 17 years of age. J
Pediatr. 1998;132(2):211-22.
Additional References
Bobo N, Evert A, Gallivan J, et al. An update on type 2 diabetes in youth from the National Diabetes Education
Program. Pediatrics. 2004;114(1):259-63
Garcia VF, Langford L, Inge TH. Application of laparoscopy for bariatric surgery in adolescents. Curr Opin Pediatr.
2003;15(3):248-55
Krebs NF, Jacobson MS; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric
overweight and obesity. Pediatrics. 2003;112(2):424-30
Inhaled steroids:
u
1000 mcg/day fluticasone (Flovent)
u
2000 mcg/day of all others
Oral Steroids:
u
20 days in previous year, or any
within 60 days of screening
u
L-asparaginase
u
FK506 (Tacrolimus)
u
Cyclosporine (Neoral/
Sandimmune)
u
Niacin
Medications known to cause wt gain:
u
Risperidone (Risperdal)
u
Olanzapine (Zyprexa)
u
Clozapine (Clozaril)
u
Quetiapine (Seroquel)
u
Ziprasidone (Geodon)
u
Carbamazepine (Tegretol)
u
Valproic acid (Depakote/
Depakene/Depacon)
u
Tricyclic Antidepressants
u
Lithium
u
Insulin/Insulin Analogs
u
Sulfonylureas
u
Cyproheptadine
u
Estrogens/Progestins
2 of 4 – Weight Management Algorithm for Overweight Children and Adolescents – Approved 04/28/05
Abbreviations
AN: Acanthosis Nigricans
CHF:
Co
ngestive Heart Failure
CVA: Cerebrovascular Accident
CVD: Cardiovascular Disease
FPG:
Fa
sting Plasma Glucose
GERD: Gastro-esophageal Reflux Disease
HCP:
He
alth Care Professional
HDL-C: High-density Lipoprotein Cholesterol
HTN: Hypertension (>95th%ile Blood Pressure for Age
& Gender & Ht)
LDL-C:
Lo
w-density Lipoprotein Cholesterol
MAOI: Monoamine Oxidase Inhibitors
NAFLD: Non-alcoholic Fatty Liver Disease
PCOS:
Polycystic Ovary Syndrome
SGA: Small for Gestational Age
SSRI:
Selective Serotonin Reuptake Inhibitors
T2DM: Type 2 Diabetes Mellitus
TG:
Tr
iglycerides
Criteria for Bariatric Surgery
8
Adolescents being considered for bariatric surgery should:
u
Have failed 6 months of organized attempts at wt
management, as determined by their primary care
provider
u
Have attained or nearly attained physiologic maturity
u
Be severely obese (BMI ≥40) with serious obesity-
related comorbidities or BMI ≥50 with less severe
comorbidities
u
Demonstrate commitment to comprehensive medical
and psychologic evaluations both before and after
surgery
u
Agree to avoid pregnancy for at least 1 yr
postoperatively
u
Be capable of and willing to adhere to nutritional
guidelines postoperatively
u
Provide informed consent to surgical treatment
u
Demonstrate decisional capacity
u
Have a supportive family environment
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

3 of 4 – Weight Management Algorithm for Overweight Children and Adolescents – Approved 04/28/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

2543 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
26
24
22
20
18
16
14
12
kg/m
2
28
26
24
22
20
18
16
14
12
kg/m
2
30
32
34
BMI
BMI
AGE (YEARS)
13
15
17
19
21
23
25
27
13
15
17
19
21
23
25
27
29
31
33
35
95
90
75
50
25
10
5
85
2 to 20 years: Boys
Body mass index-for-age percentiles
NAME
RECORD #
SOURCE: Developed b
(2000).
y the National Center for Health Statistics in collaboration with
the National Center for Chronic Disease Prevention and Health Promotion
http://www.cdc.gov/growthcharts
Date Age Weight Stature BMI*
Comments
Published May 30, 2000 (modified 10/16/00).
2 to 20 years: Girls
Body mass index-for-age percentiles
NAME
RECORD #
SOURCE: Developed b
(2000).
y the National Center for Health Statistics in collaboration with
the National Center for Chronic Disease Prevention and Health Promotion
http://www.cdc.gov/growthcharts
2543 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
26
24
22
20
18
16
14
12
kg/m
2
28
26
24
22
20
18
16
14
12
kg/m
2
30
32
34
BMI
BMI
AGE (YEARS)
13
15
17
19
21
23
25
27
13
15
17
19
21
23
25
27
29
31
33
35
Date Age Weight Stature BMI*
Comments
95
90
85
75
50
10
25
5
Published May 30, 2000 (modified 10/16/00).
4 of 4 – Weight Management Algorithm for Overweight Children and Adolescents – Approved 04/28/05 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Exercise Algorithm
Type 2 Diabetes Prevention and Therapy
If your patients are “apparently healthy” and have fewer than two major risk
factors for cardiovascular disease (
C
VD
), t
hen they are categorized by age.
u
For men and women under 35 yrs. of age, there are no limitations. They
can safely begin or continue a program of moderate or vigorous activity.
u
If they exceed the age limit (≥35 yrs.), it is safe to limit your
recommendations to moderate activity (55% to 70% maximum heart rate)
for both genders.
Pat
ients in this group who wish to participate in vigorous
or competitive activities should be considered for an
E
TT
s
creening.
If you
r patients have one or more major risk factors for cardiovascular disease,
they should undergo an
E
TT
b
efore beginning a moderate exercise program.
It is i
mportant to underscore the fact that the majority of your patients,
regardless of risk factors, can and should be encouraged to start or continue a
program of regular moderate physical activity.
Yes
Age <35 yrs.
Impaired Fasting Plasma Glucose
100−125 mg/d
L
Screening/Risk Assessments
Minimum Standards for Diabetes Care in Texas
Proper Footwear*
Diagnosis Diabetes Mellitus
F
PG ≥1
26 mg/d
L
Consider ETT
1
Prio
r to
Pre
scription
Low intensity/
Low i
mpact activity
2
Low intensity/
Low i
mpact activity
2, 3
CardiovascularDisease (CVD)
Proliferative Retinopathy
Vascular/Orthopedic Peripheral
Neu
ropathy
One or more major risk factors:
Hypertension
Smok
ing
Hyperlipidemia
Family history of
C
VD
Yes
Yes
Low intensity/
Low i
mpact activity
2
ETT is recommended prior to
moderate or vigorous activity
1
Age ≥35 yrs.
Moderate or
vigorous activity
Moderate physical
activity
ETT recommended
before vigorous
physical activity
1
YesNo
1
Recommendation for Exercise Tolerance Test
Bas
ed on the clinical context in which they occur, if your patients have any
of the following signs or symptoms of cardiovascular or metabolic disease,
consider an exercise tolerance test (
ET
T
) b
efore recommending moderate
or vigorous activity.
u
Pain, discomfort (or other anginal equivalent) in the chest, neck, jaw,
arms, or other areas that may be ischemic in nature
u
Shortness of breath at rest or with mild exertion
u
Dizziness or syncope
u
Orthopnea or paroxysmal nocturnal dyspnea
u
Ankle edema
u
Palpitations or tachycardia
u
Intermittent claudication
u
Unusual fatigue or shortness of breath with usual activities
u
Any macrovascular disease
u
Any microvascular disease
u
Peripheral vascular disease
2
Moderate activity is recommended to achieve physiologic improvement.
3
Orthotics as indicated.
*
Pro
per footwear (socks, shoes, insoles) to prevent injury.
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
1 of 2 – Exercise Algorithm Type 2 Diabetes Prevention and Therapy – Revised 01/22/04
Revised 01/22/04Publication # 45-11266

CONSIDERATIONS FOR PRESCRIBING PHYSICAL ACTIVITY FOR
TYPE 2 DIA
BETES PREVENTION AND
TRE
ATMENT
Significant health benefits can be obtained by including an accumulated 30 minutes of moderate
physical activity on most, if not all, days of the week.
Regular physical activity lowers the risk of developing type 2 diabetes – 1996 Surgeon General’s
Report on Physical Activity and Health.
“Regular physical activity” includes all movements in everyday life, including work, recreation,
exercise, and sporting activities.
• Low Intensity/Low Impact Activity – includes activities like walking, housework, light
gardening, light yard work, and social dancing
• Moderate Intensity Activity – includes activities like brisk walking, vigorous gardening,
slow cycling, aerobic dancing, doubles tennis, or hard work around the house
PRECAUTIONS FOR EXERCISE PRESCRIPTION
Retinopathy
Patients with proliferative diabetic retinopathy have abnormal hemodynamic responses of
the cerebral and ophthalmic circulation both at rest and with exercise. Vigorous physical
activity, especially isometric contractions, produces significant increases in blood pressure
and can accelerate proliferative diabetic retinopathy with significant risk of retinal and vitreal
hemorrhage and detachment. Low impact/low intensity physical activity recommended.
Orthopedic Problems
Neuropathy and peripheral vascular disease can predict unnoticed foot injury. Footwear that
relieves forefoot plantar pressure by up to 50% has been shown to be effective in preventing
the recurrence of foot ulcers when worn for more than 60% of the day (Peirce, N. 1999. British
Journal of Sports Medicine).
Guidelines for Exercise Prescription
1. Appropriate attire for physical activity, i.e., footwear – socks, shoes, insoles/orthotics
2. Do not exercise at peak hypoglycemic times
3. Monitor blood glucose before and during exercise if symptoms of hypoglycemia
occur with exercise
4. Wear a form of personal identification or medical alert
5. Carry fast-acting carbohydrate, i.e., sucrose and glucose products
6. Examine feet after exercise
7. Maintain adequate hydration
Diabetes treatment algorithms
2 of 2 – Exercise Algorithm Type 2 Diabetes Prevention and Therapy – Revised 01/22/04 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes Medical Nutrition Therapy
and Prevention Algorithm For Adults
Diabetes Mellitus
u
Fasting glucose 126 ≥ mg/dL or
u
2 hr. PP ≥ 200 mg/dL or
u
Alc ≥6.5%
Medical Nutrition Therapy
Recommend Registered/Licensed Dietitian or Certified Diabetes Educator with Experience in Diabetes Nutrition Counseling
Overweight/Obesity
2, 3
BMI >25
u
Set weight loss
goals (5–10%
minimum)
u
↑ physical activity
BP >130/80 mmHg
6
u
Sodium restriction to
≤ 1.
5g/day
7
u
DASH diet
If proteinuric:
u
Maintain protein intake
between 0.8–1g/kg
u
Continue sodium
restriction
u
Evaluate total calories from fat and CHO.
u
If excessive, ↓ Kcal from CHO and/or fat,
(especially from saturated fat) by 500-1000
Kcal below usual daily intake.
TG >150 mg/dL
5
u
↓ Saturated fat < 7%
to
tal Kcal/day
u
↓ trans fats (minimized)
u
↓ cholesterol intake
< 20
0 mg/day
u
↑ omega-3 fatty acids
u
↑ soluble fiber
10
–25g/day
u
↑ plant stenols / sterols
LDL-C >100 mg/dL
5
u
↓ Total CHO intake
u
↑ Omega-3 fatty acids
Monitor glucose (SMBG), A1c, weight, lipids, and blood pressure. Adjust food portions and distribution with medication and activity to achieve metabolic goals.
Monitor glucose (SMBG), A1c, weight, lipids, and blood pressure. Modify meal plan as needed to achieve metabolic goals.
If metabolic targets are not met within 1–3 months, evaluate nutrition care plan, re-educate and review goals.
Ver
ify patient follow-up with healthcare provider for further assessment and treatment.
1
This test requires the use of a glucose load containing the
equivalent of 75 g anhydrous glucose dissolved in water.
2-hr post-challenge glucose.
2
Refer to Weight Loss Algorithm
3
Refer to Exercise Algorithm
4
ADA. Standards of Medical Care in Diabetes – 2010.
Diabetes Care. 2010;33 (suppl 1):
S11
-
S61
.
5
Refer to Lipid Treatment Algorithm
6
Refer to Hypertension Algorithm
7
Dietary Guidelines for Americans, 2005. Available online at
h
ttp://www.health.gov/dietaryguidelines/dga2005/
document/html/chapter8.htm Accessed on July 22, 2010.
If TG >500 mg/dL,
↓ fat calories to <15%
total Kcal/day
u
Fasting glucose>110 mg/dL
u
2-hr PP ≥140–180 mg/dL
u
A1c >6.5%
u
Monitor total CHO intake
u
Refer to Glycemic Control
Algorithm
Follow-up Evaluation
Categories of Increased Risk
u
IFG: Fasting glucose 100–125mg/dL
1
or
u
IGT: Post challenge glucose 140–199mg/dL or
u
Alc: 5.7– 6.4%
Diabetes Self-Management Education (DSME)
u
Set individualized goals to meet patient needs
u
Promote weight loss, if needed
2
u
Self-monitored blood glucose (SMBG)
u
Increase physical activity
3
u
Incorporate other needed dietary modifications with meal plan
u
Meal plan
4
– Distribute food throughout the day to avoid large
concentrations of calories or carbohydrates that cause postprandial
glucose elevations.
R
ecommended: low
CHO d
iet, no more than 45–65% Kcal from
CHO
Not Recommended: <130g CHO (~9 servings/day)
u
Individualize meals and snacks to include healthy food choices
u
↑ dietary fiber intake to 14 g fiber/1,000 Kcal /day
Footnotes
Individual Nutrition Assessment
BMI, Waist Circumference/Waist-to-hip ratio, Medical History, Lab Values (Chemistry panel, Lipid panel, A1c,
microalbumin-to-creatinine ratio),
Die
t History,
Lif
estyle,
Phy
sical Activity,
Rea
diness to
Change
Interventions
Abbreviations:
A1c – Hemoglobin A1c
BMI – Bod
y Mass
Ind
ex
BP – Blo
od
Pre
ssure
CHO – Carbohydrates
DASH –
Die
tary Approaches
to
Sto
p Hypertension
IFG
–
Impa
ired Fasting
Glucose
IGT – Impaired Glucose
Tole
rance
LD
L
-C – Low Den
sity
Lipo
protein
Chol
esterol
PP – Pos
tprandial
TG
–
Trig
lycerides
Diabetes treatment algorithms
Revised 07/22/10Publication # 45-10778
1 of 1 – Diabetes Medical Nutrition Therapy and Prevention Algorithm for Adults – Revised 07/22/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
Medical nutrition therapy (MNT) is important in preventing diabetes, managing
existing diabetes, and preventing, or at least slowing, the rate of development of diabetes
complications. It is an integral component of diabetes self-management education (or
training). The following recommendations and interventions are evidence-based.
The goal of these recommendations is to make people with diabetes and health care
providers aware of beneficial nutrition interventions. This requires the use of the best
available scientific evidence while taking into account treatment goals, strategies to attain
such goals, and changes individuals with diabetes are willing and able to make. Achieving
nutrition-related goals requires a coordinated team effort that includes the person with
diabetes and involves him or her in the decision-making process. It is recommended that
a registered dietitian, knowledgeable and skilled in MNT, be the team member who
plays the leading role in providing nutrition care. However, it is important that all team
members, including physicians, certified diabetes educators, nurses, pharmacists and other
providers, be knowledgeable about MNT and support its implementation.
Goals: At risk for diabetes or with pre-diabetes
1) To decrease the risk of diabetes and cardiovascular disease (CVD) by promoting healthy
food choices and physical activity leading to moderate weight loss that is maintained.
Goals: Individuals with diabetes
1) Achieve and maintain
· Blood glucose levels in the normal range or as close to normal as is safely possible
·
A lipid
and lipoprotein profile that reduces the risk for cardiovascular disease
·
Blood
pressure levels in the normal range, less than 130/80
2)
T
o prevent, or at least slow, the rate of developing complications of diabetes by
modifying nutrient intake and lifestyle
3)
To
address individual nutrition needs, taking into account personal and cultural
preferences and willingness to change
4) To maintain the pleasure of eating by only limiting food choices when indicated by
scientific evidence
Goals: Specific Situations
1) For youth with type 1 diabetes, youth with type 2 diabetes, pregnant and lactating
women, and older adults with diabetes, to meet the nutritional needs of these unique
times in the life cycle.
2) For individuals treated with insulin or insulin secretagogues, to provide self-management
training for safe conduct of physical activity, including the prevention and treatment of
hypoglycemia and diabetes treatment during acute illness.
Nutrition Recommendations and
Interventions for Diabetes
1 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement
sUPPlement
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
EFFECTIVENESS of Medical Nutrition Therapy
Recommendations
u
Individuals who have pre-diabetes or diabetes should receive Individualized MNT;
such therapy is best provided by a registered dietitian familiar with the components
of diabetes MNT.
u
Nutrition counseling should be sensitive to the personal needs, willingness to
change, and ability to make changes of the individual with pre-diabetes or diabetes.
Reference: Diabetes Care. 2007 Jan;30 Suppl 1:S48-65.
A. Nutrition Guidelines
1. Stress consistent timing of meals, snacks, and portion control. Review the number of
servings needed per meal and snacks.
2.
Eat a
variety of foods every day including fruits and vegetables.
3.
Achiev
e or maintain a desirable weight.
4.
Reduce
total calories if overweight or obese to lose weight.
5.
Read
nutrition facts labels.
6.
Eat foods
high in fiber (whole grain products, vegetables, raw fruit, beans, and legumes).
7.
Eat the
least amount of saturated fats and trans fats.
B. Carbohydrate (CHO) Intake
Low carbohydrate diets, restricting total CHO to less than 130 grams per day, are not
recommended.
1. Total grams of carbohydrate should be individualized based on glucose control,
medication and physical activity.
2.
Consume mor
e complex (unrefined) carbohydrates with fiber.
3.
Eat 2
servings of fruits each day, preferably with lunch and dinner. One serving equals:
½ c. canned fruit or juice, or 1 c. fresh fruit. Avoid juices (except when hypoglycemic)
which may cause the blood glucose to rise very rapidly. Focus on fresh fruits that have
more fiber, but no more than 2–3 servings per day.
4.
Eat 4–6
servings of non-starchy vegetables each day. One serving equals: ½ c. cooked
vegetable, ½ c. vegetable juice, or 1 c. raw vegetable.
5.
Other
CHO choices include: 1 tortilla, 1 slice of bread, 1/3 c. cooked pasta, rice,
garbanzo beans, ½ c corn, peas, potatoes, beans, or 6 saltine crackers. Limit CHO
choices to 2–3 per meal.
6.
Sucrose containing foods can be substituted for other CHO choices in the meal plan, if
added to the meal plan.
2 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
C. Fiber Intake
1. Eat 14 grams per 1,000 calories. Example: 22 grams for 1,500 calories, 28 grams for
2,000 calories a day.
2.
Major
sources: raw fruits, unpeeled vegetables, beans, legumes, whole grain breads,
pastas, and fiber-rich cereals (≥ 5 grams per serving).
D. Protein Intake
1. 15-20% of total calories per day; approximately 4-6 ounces per day (3 oz. = the size of a
deck of cards).
2.
Restrict
to 0.8–1.0 gram protein/kg of body weight for adults with onset of early
nephropathy. Restrict to 0.8gram protein/kg of body weight for adults with onset of later
stages of nephropathy
3. One serving is: 1 oz. lean beef, chicken, turkey, pork, lamb or fish, 1 c. skim milk, yogurt,
1 oz. cheese, 1 egg, 1 T. peanut butter
4.
Adjustments
should be made for conditions such as renal failure, hypertension, or
hyperlipidemia.
E. Fat Intake
1. Limit dietary cholesterol to less than 200 mg per day
2.
Limit saturated
fat to less than 7% of total calories per day
Sources: Animal
fats (found in fatty meats, poultry skin, hydrogenated shortenings and
fats, some vegetable oils (coconut, palm, palm kernel, cocoa butter), whole milk, whole
milk products, butter, and most commercially baked products.
3.
Minimum
intake of trans fatty acids (found in most commercially baked products)
4.
Use
more mono-unsaturated fats, i.e., olive oil and poly-unsaturated fats, i.e., canola or
corn oils.
5. Two or more servings of fish per week (with the exception of commercially fried filets)
F. Alcohol (Use with doctor’s approval)
1. Limited to a moderate amount (less than 1 drink per day for adult women and less than
2 drinks per day for adult men).
2. One drink is: 1.5 oz. distilled spirits, 5 oz. wine or 12 oz. beer.
3. Food should be consumed with alcoholic beverages to prevent hypoglycemia.
G. Reduced Calorie Sweeteners
Nonnutritive Sweeteners:
1. Acesulfame potassium
2. Aspartame
3 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
3. Neotame
4.
Saccharin
5. Sucralose
Nutritive Sweeteners:
1.
Glucose,
dextrose, corn syrup
2.
Fr
uctose (fruit sugar), molasses, lactose
3.
Honey
, raw honey, invert sugar
4. Maltose, malted syrup, dextrin
Sugar Alcohols (Polyols):
1. Erythritol, isomalt, lactitol, maltitol, mannitol, sorbitol, xylitol, tagatose, and hydrogenated
starch hydrolysates.
H. Sodium
u
In normotensive and hypertensive individuals, a reduced sodium intake (e.g., 2,300 mg per
day with a diet high in fruits, vegetables, and low-fat dairy products lowers blood pressure.
u
Individuals with diabetes at risk for CVD, diets high in fruits, vegetables, whole grains, and
nuts may reduce the risk.
u
Individuals with diabetes and symptomatic heart failure, dietary sodium intake of <2,000
mg. per day may reduce symptoms.
u
In most individuals, a modest amount of weight loss beneficially affects blood pressure.
u
Choose low-sodium foods: fresh or frozen vegetables (avoid regular canned foods) and
powdered seasonings with sodium (avoid onion and garlic salt). Avoid salty sauces such
as soy sauce. Eat less fast food and convenience foods, these foods contain high levels of
sodium.
4 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
5 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
6 of 6 – Nutrition Recommendations and Interventions for Diabetes – Supplement
10
2
3
5
8
9
7
6
4
1
switch to fat-free or
low-fat (1%) milk
They have the same amount of
calcium and other essential nutrients as
whole milk, but fewer calories and less
saturated fat.
make half your grains whole grains
To eat more whole grains, substitute a whole-grain
product for a rened product—such as eating whole-
wheat bread instead of white bread or brown rice instead of
white rice.
foods to eat less often
Cut back on foods high in solid fats, added sugars,
and salt. They include cakes, cookies, ice cream,
candies, sweetened drinks, pizza, and fatty meats like ribs,
sausages, bacon, and hot dogs. Use these foods as
occasional treats, not everyday foods.
compare sodium in foods
Use the Nutrition Facts label
to choose lower sodium versions
of foods like soup, bread, and frozen
meals. Select canned foods labeled
“low sodium,” ”reduced sodium,” or
“no salt added.”
drink water instead of sugary drinks
Cut calories by drinking water or unsweetened
beverages. Soda, energy drinks, and sports drinks
are a major source of added sugar, and calories, in American
diets.
balance calories
Find out how many calories YOU need for a day
as a rst step in managing your weight. Go to
www.ChooseMyPlate.gov to nd your calorie level. Being
physically active also helps you balance calories.
enjoy your food, but eat less
Take the time to fully enjoy
your food as you eat it. Eating
too fast or when your attention is
elsewhere may lead to eating too
many calories. Pay attention to hunger
and fullness cues before, during, and after meals. Use
them to recognize when to eat and when you’ve had
enough.
avoid oversized portions
Use a smaller plate, bowl, and glass. Portion out
foods before you eat. When eating out, choose a
smaller size option, share a dish, or take home part of
your meal.
foods to eat more often
Eat more vegetables, fruits, whole grains, and fat-free
or 1% milk and dairy products. These foods have the
nutrients you need for health
—
including potassium, calcium,
vitamin D, and ber. Make them the
basis for meals and snacks.
make half your plate
fruits and vegetables
Choose red, orange, and dark-green vegetables like
tomatoes, sweet potatoes, and broccoli, along with other
vegetables for your meals. Add fruit to meals as part of
main or side dishes or as dessert.
choose MyPlate
10 tips to a great plate
Making food choices for a healthy lifestyle can be as simple as using these 10 Tips.
Use the ideas in this list to balance your calories, to choose foods to eat more often, and to cut back on foods
to eat less often.
DG TipSheet No. 1
June 2011
Center for Nutrition USDA is an equal opportunity
Policy and Promotion provider and employer.
Go to www.ChooseMyPlate.gov for more information.
10
tips
Nutrition
Education Series
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Glycemic Control Algorithm For
Type 2 Diabetes Mellitus In Adults
Glycemic Goals
1
Individualize goal based on
patient risk factors
A1c
≤6% <7
%
<
8%
FPG ≤1
10
12
0
14
0 mg/d
L
2h P
P
≤1
30
18
0
18
0 mg/d
L
Initial Intervention
2
1. Diabetes Self-Management Education and
2. Self-monitored Blood Glucose
3
and
3.
Medical
Nut
rition
3
, Weight Control
3
, Exercise
3
and
4. Monotherapy if A1c <1% above goal otherwise Dual
Thera
py (optimize therapy as tolerated)
If A1c < 1% above goal:
u
If on monotherapy → add second agent (oral or GLP-1)
u
If on dual therapy → add third agent (oral or GLP-1 or insulin
6
)
If A1c ≥ 1% above goal:
u
If on monotherapy → add second agent +/- once-daily insulin
6
OR add two non-insulin agents (oral or GLP-1)
u
If on dual therapy → add third agent (oral or GLP-1)
O
R
ad
d insulin
6
Continue Therapy
A1c every 3-6 months
Add or intensify insulin
6
Consider referral to endocrinologist / diabetes specialist
Goals not met after 3 months of optimized therapy
Goals not met after 3 Months of optimized therapy
Goals Achieved
Recommended Options for Dual Therapy
4
Metformin
+ TZD or DPP-4 or SU
5
or GLP-1 or
Me
glitinide or colesevelam
Recommended Options for Triple Therapy
Metformin
+
T
ZD o
r
S
U
5
+ GLP-1 o
r
D
PP
-4 o
r AG
I o
r colesevelam
Metformin
+
T
ZD o
r
D
PP
-4 o
r AG
I o
r
S
U
5
or colesevelam
+ Insul
in
Abbreviations
AG
I Alp
ha-glucosidase inhibitors
DPP-4 Dipeptidyl peptidase-4 Inhibitor
F
PG Fa
sting plasma glucose
G
LP-1 Gl
ucagon-like peptide-1 agonist
PP
P
ostp
randial
SU
S
ul
fonylurea
TZD Thi
azolidinedione
Footnotes
1
Intensify management if: Absent/stable cardiovascular disease, mild-moderate microvascular
complications, intact hypoglycemia awareness, infrequent hypoglycemic episodes, recently diagnosed
diabetes. Less intensive management if:
Evi
dence of advanced or poorly controlled cardiovascular
and/or microvascular complications, hypoglycemia unawareness, vulnerable patient (ie, impaired
cognition, dementia, fall history).
Ref
er to
T
DC
“A
1c Goal” treatment strategy for further explanation.
A1c is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA Clinical Practice
Rec
ommendations. Diabetes Care 2010;33(suppl 1):
S19
-20.
2
If initial A1c on presentation is ≥10%, consider the use of insulin, with or without oral agents, as the initial
intervention (see
Ins
ulin Algorithm).
Oth
er agents may be introduced as glycemic control improves.
If
k
etoacidosis or recent rapid weight loss, consider
Typ
e 1 diagnosis.
3
These interventions should be maintained life-long; (refer to Medical Nutrition, Weight Loss, and Exercise
Algorithms).
4
Refer to the Diabetes Medications Supplement: Working Together to Manage Diabetes found in the Texas
Dia
betes
Coun
cil’s
Dia
betes
Too
lkit.
5
If a SU is selected, low dose glipizide ER or glimepiride are recommended because they have a lower
incidence of hypoglycemia than glyburide.
6
Refer to Insulin Algorithm for Type 2 Diabetes Mellitus in Children and Adults / Initial Insulin Therapy for
Ty
pe 2
Dia
betes Mellitus in
Chi
ldren and Adults: A
Sim
plified Approach
Revised 07/22/10Stock # 45-11265
1 of 3 – Glycemic Control Algorithm for Type 2 Diabetes Mellitus nn Adults – Revised 07-22-10
Diabetes treatment algorithms
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Recent Review Articles
Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review.
JAMA. 2002;287(3):360-72.
Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: Incretin-
based therapy and beyond. Circulation. 2008 Jan 29;117(4):574-84.
Riddle, MC. Glycemic management of type 2 diabetes: An emerging strategy with oral
agents, insulins and combinations. Endocrinol Metab Clin N Am. 2005;34(1):77-98.
Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-
based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits.
Diabetes Care. 2010 Feb;33(2):428-33.
Dual Therapy
Metformin or Sulfonylurea + Acarbose
Chiasson JL, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of
patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical
trial. Ann Intern Med. 1994;121(12):928-35.
Metformin + Pioglitazone
Einhorn D, Rendell M, Rosenzweig J, et al. Pioglitazone hydrochloride in combination
with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-
controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22(12):1395-409.
Metformin + Rosiglitazone
Fonseca V, Rosenstock J, Patwardhan R, et al. Effect of metformin and rosiglitazone
combination therapy in patients with type 2 diabetes mellitus: a randomized controlled
trial. JAMA. 2000;283(13):1695-702. Erratum in: JAMA 2000;284(11):1384.
Sulfonylurea + Pioglitazone
Kipnes MS, Krosnick A, Rendell MS, et al. Pioglitazone hydrochloride in combination
with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes
mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111(1):10-7.
Sulfonylurea + Rosiglitazone
Wolffenbuttel BH, Gomis R, Squatrito S, et al. Addition of low-dose rosiglitazone to
sulphonylurea therapy improves glycaemic control in type 2 diabetic patients. Diabet
Med. 2000;17(1):40-7.
Metformin or Sulfonylurea + Exenatide
Buse JB, Henry RR, Han J, et.al. Effects of exenatide (exendin-4) on glycemic control
over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care.
2004;27(11):2628-35.
DeFronzo RA, Ratner RE, Han J, et.al. Effects of exenatide (exendin-4) on glycemic
control and weight over 30 weeks in metformin-treated patients with type 2 diabetes.
Diabetes Care. 2005;28(5):1092-100.
Nateglinide or Repaglinide + Metformin
Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide
plus metformin versus nateglinide plus metformin. Diabetes Care. 2003;26(7):2063-8.
Erratum in: Diabetes Care. 2003;26(9):2708.
Repaglinide + Metformin
Moses R, Slobodniuk R, Boyages S, et al. Effect of repaglinide addition to metformin
monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care.
1999;22(1):119-24.
Nateglinide + Metformin
Horton ES, Clinkingbeard C, Gatlin M, et al. Nateglinide alone and in combination
with metformin improves glycemic control by reducing mealtime glucose levels in type 2
diabetes. Diabetes Care. 2000;23(11):1660-5.
Nateglinide + Thiazolidinedione
Rosenstock J, Shen SG, Gatlin MR, et al. Combination therapy with nateglinide
and a thiazolidinedione improves glycemic control in type 2 diabetes. Diabetes Care.
2002;25(9):1529-33.
Fonseca V, Grunberger G, Gupta S, et al. Addition of nateglinide to rosiglitazone
monotherapy suppresses mealtime hyperglycemia and improves overall glycemic control.
Diabetes Care. 2003;26(6):1685-90.
Repaglinide + Thiazolidinedione
Raskin P, Jovanovic L, Berger S, et al. Repaglinide/troglitazone combination therapy:
improved glycemic control in type 2 diabetes. Diabetes Care. 2000;23(7):979-83.
Liraglutide + Metformin
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M,
Düring M, Matthews DR; LEAD-2 Study Group.Efficacy and safety comparison of
liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2
diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care.
2009 Jan;32(1):84-90.
Liraglutide + Sulfonylurea
Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic
M, Le Thi TD, Colagiuri S; LEAD-1 SU study group. Liraglutide, a once-daily human
GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements
in glycaemic and weight control compared with adding rosiglitazone or placebo in
subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009 Mar;26(3):268-78.
Triple Therapy
Sulfonylurea + Metformin + Alpha glucosidase inhibitors
Lam KS, Tiu SC, Tsang MW, et al. Acarbose in NIDDM patients with poor control
on conventional oral agents. A 24-week placebo-controlled study. Diabetes Care.
1998;21(7):1154-8.
Standl E, Schernthaner G, Rybka J, et al. Improved glycaemic control with miglitol in
inadequately-controlled type 2 diabetics. Diabetes Res Clin Pract. 2001;51(3):205-13.
Sulfonylurea + Metformin + Thiazolidinedione
Dailey GE 3rd, Noor MA, Park JS, et al. Glycemic control with glyburide/metformin
tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized,
double-blind trial.
Am J Med. 2004;116(4):223-9.
GLYCEMIC CONTROL BIBLIOGRAPHY
2 of 3 – Glycemic Control Algorithm for Type 2 Diabetes Mellitus nn Adults – Revised 07-22-10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Aljabri K, Kozak SE, Thompson DM. Addition of pioglitazone or bedtime insulin to
maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor
glucose control: a prospective, randomized trial. Am J Med. 2004;116(4):230-5.
Sulfonylurea + Metformin + Exenatide
Kendall DM, Riddle MC, Rosenstock J, et.al. Effects of exenatide (exendin-4) on
glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin
and a sulfonylurea. Diabetes Care. 2005;28(5):1083-91.
Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin
glargine in patients with suboptimally controlled type 2 diabetes: a
randomized study. Ann Intern Med. 2005; 143(8):559-69.
Liraglutide + Metformin and TZD
Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M,
Blonde L; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like
peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in
patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009 Jul;32(7):1224-
30.
Liraglutide + Metformin and Sulfonylurea
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn
GM, Simó R; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study
Group. Liraglutide vs insulin glargine and placebo in combination with metformin
and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised
controlled trial. Diabetologia. 2009 Oct;52(10):2046-55.
Colesevelam
Welchol
TM
Prescribing Information. Daiichi Sankyo, Inc. October 2009.
Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in
patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects.
Arch Intern Med. 2008 Oct 13;168(18):1975-83.
Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in
patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-
based therapy. Arch Intern Med. 2008 Jul 28;168(14):1531-40.
GLYCEMIC CONTROL BIBLIOGRAPHY (CONT.)
3 of 3 – Glycemic Control Algorithm for Type 2 Diabetes Mellitus nn Adults – Revised 07-22-10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Hypertension Algorithm
for Diabetes in Adults
BP ≤130/80 mmHg BP>130/80 mmHg
Follow-up BP each visit
If microalbuminuria or nephropathy
present (Table 1)
Continue Therapy
BP Check Every Visit
Footnotes
1
Joint National Committee on Detection, Evaluation and Treatment of High
Blood Pressure: The seventh report of the Joint National Committee on
Detection, Evaluation and Treatment of High Blood Pressure (JNC 7). JAMA.
2003;289(19):2560-72; consider secondary causes as appropriate
2
Maintain non-pharmacological therapy throughout treatment. Medical
Nutrition Therapy Algorithm + low sodium diet (<2.4 g/day; if ≥ age 50,
≤ 1.5 g/day) + limit alcohol intake (1 oz./day for men, 0.5 oz./day for
women) Weight Loss and Exercise Algorithms.
3
ADA Clinical Practice Guidelines 2004. Diabetes Care. 27(suppl 1):S15-S35,
S65-S68.
4
Monitor serum K
+
and creatinine periodically
5
If intolerant to ACEi (except angioedema) consider
angiotensin receptor blocker (ARB).
6
Am J Kids Dis. 2000;36:646-61
7
Metoprolol, carvedilol, bisoprolol, atenolol
8
Amlodipine, felodipine, isradipine, nicardipine,
nisoldipine
Table 1
Microalbuminuria/Proteinuria
3
u
In Type 2 patients, an ACEi or angiotensin
receptor blocker (ARB) may be used first
line.
u
In Type 1 patients, an ACEi is recommended
to reduce protein excretion
u
Consider the use of verapamil or diltiazem in
patients with proteinuria unable to tolerate
ACEi or ARBs.
Assess Blood Pressure
1
(BP)
Start ACE inhibitor (ACEi) therapy
2-5
IF microalbuminuria or nephropathy present (Table 1)
IF
A
frican-American- Start ACEi in combination with diuretic or CCB
IF
S
BP ≥145mmHg and/or DBP≥90mmHg
6
Start with combination antihypertensive therapy
Reassess therapy in 4-8 weeks-Titrate to at least 1/2 max dose
Encourage self-monitoring of blood pressure
1
(on average ≥ 3 medications will be needed to achieve blood pressure goals)
Add
6
Diuretic OR Calcium Channel Blocker (CCB) OR Beta Blocker
If Diuretic Chosen: (Preferred if no other compelling indications)
Creatinine <1.8 mg/dL
Cr
eatinine ≥1.8 mg/dL
Thiazide diuretic^
L
oop Diuretic
(^ Max. dose 25mg Hydrochlorothiazide or equivalent)
If Beta Blocker Chosen: (Strongly recommended if history of MI) Choose beta blocker without intrinsic
sympathomimetic activity
7
If CCB Chosen:
I
f Diltiazem or Verapamil Chosen: Pulse and conduction effects should be considered if combined
with b blocker
If Dihydropyridine CCB8 Chosen: Not to be used without ACEi or ARB agents
Reassess therapy in 4-8 weeks
Titrate to at least 1/2 max dose or add additional agent
6
DBP Diastolic Blood Pressure
MI
M
yocardial Infarction
SBP
S
ystolic Blood Pressure
BP ≤130/80 mmHg BP>130/80 mmHg
BP ≤130/80 mmHg BP>130/80 mmHg
BP ≤130/80 mmHg
BP>130/80 mmHg
Refer to Specialist (Endocrinologist or Nephrologist)
OR
ADD: a blocker, hydralazine, clonidine (caution with b blocker)
***Alternative treatment
BP >130/80 mmHg despite above agents or
if intolerance/contraindications exist:
ADD: Medication not chosen from above
OR Go to Alternative Treatment***
Revised 1/26/12Publication # 45-11267
1 of 2 – Hypertension Algorithm for Diabetes in Adults – Revised 1/26/12 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Proper blood pressure assessment
National Committee on Detection, Evaluation and Treatment
of High Blood Pressure: The Seventh Report of the Joint National
Committee on Detection, Evaluation and Treatment of High Blood
Pressure (JNC 7). National Institutes of Health, National Heart,
Lung and Blood Institute, 2003 http://www.nhlbi.nih.gov/
guidelines/hypertension/
ACE inhibitor as 1t line therapy in Diabetes Mellitus
National Committee on Detection, Evaluation and Treatment
of High Blood Pressure: The Seventh Report of the Joint National
Committee on Detection, Evaluation and Treatment of High Blood
Pressure (JNC 7). National Institutes of Health, National Heart,
Lung and Blood Institute, 2003 http://www.nhlbi.nih.gov/
guidelines/hypertension/
Kasiske BL, Kalil RS, Ma JZ, et al.: Effect of antihypertensive
therapy on the kidney in patients with diabetes: a meta-regression
analysis. Ann Intern Med 118:129–38, 1993
UK Prospective Diabetes Study Group: Efficacy of atenolol and
captopril in reducing the risk of macrovascular complications in
type 2 diabetes (UKPDS 39) BMJ 317:713–20, 1998
The Heart Outcomes Prevention Evaluation Study. Effects of
an ACE inhibitor, ramipril, on cardiovascular events in high risk
patients. N Engl J Med 342:145–53, 2000
Pahor M, Psaty BM, Alderman MH, et al. Therapeutic benefits of
ACE inhibitors and other antihypertensive drugs in patients with
type 2 diabetes. Diabetes Care 23:888-
9
2, 2000
Wing LMH, Reid CM, Ryan P, et al. A comparison of outcomes
with angiotensin-converting-enzyme inhibitors and diuretics for
hypertension in the elderly (ANBP2). N
E
ngl J Med 348:583-92,
2003
Diuretic as second line
National Committee on Detection, Evaluation and Treatment
of High Blood Pressure: The Seventh Report of the Joint National
Committee on Detection, Evaluation and Treatment of High Blood
Pressure (JNC 7). National Institutes of Health, National Heart,
Lung and Blood Institute, 2003
http://www.nhlbi.nih.gov/guidelines/hypertension/
Antihypertensive & Lipid Lowering Treatment to Prevent Heart
Attack (ALLHAT) JAMA 288:2981-97, 2002
Beta-Blocker as second line
National Committee on Detection, Evaluation and Treatment
of High Blood Pressure: The Seventh Report of the Joint National
Committee on Detection, Evaluation and Treatment of High Blood
Pressure (JNC
7
). National Institutes of Health, National Heart,
Lung and Blood Institute, 2003
http://www.nhlbi.nih.gov/guidelines/hypertension/
UK Prospective Diabetes Study Group: Efficacy of atenolol and
captopril in reducing the risk of macrovascular complications in
type 2 diabetes
(UKPDS 39) BMJ 317:713–20, 1998
Hansson L, Lindholm LH, Niskanen L, et al. Effect of
angiotensin converting-enzyme inhibition compared with
conventional therapy on cardiovascular morbidity and mortality
in hypertension: the Captopril Prevention Project (CAPPP)
randomised trial. Lancet 353: 611–16, 1999
Verapamil or Diltiazem
Hansson L, Hedner T, Lund-Johansen P, et al. Randomized
trial of effects of calcium antagonists compared with diuretics
and beta-blockers on cardiovascular morbidity and mortality in
hypertension. NORDIL. Lancet 356:359–65, 2000
Bakris GL, Copley JB, Vicknair N, et al. Calcium channel
blockers versus other antihypertensive therapies on progression of
NIDDM associated nephropathy. Kidney Int 50:1641–50, 1996
Dihydropyridine calcium
channel blockers
Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effect of
calcium channel blockage in older patients with diabetes and
systolic hypertension. N Engl J Med 340:677–84, 1999
Dahlof B, Sever P, Poulter N, et al. Prevention of cardiovascular
events with an antihypertensive regimen of amlodipine
adding perindopril as required versus atenolol adding
bendroflumethiazide as required, in the Anglo-Scandinavian
Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-
BPLA): a multicentre randomised controlled trial. Lancet 366:
895-906, 2005
Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine
as compared with enalapril on cardiovascular outcomes in
patients with non-insulin-dependent diabetes and hypertension.
N Engl J Med 338:645–52, 1998
Alpha-Blockers
Major cardiovascular events in hypertensive patients randomized
to doxazosin vs chlorthalidone. (ALLHAT Data) JAMA
283:1967–75, 2000
Blood Pressure Goal <130/80
American Diabetes Association: Clinical Practice
Recommendations 2004. Diabetes Care 27 (suppl 1):S15-S35;
S65-S67, 2004
Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive
blood-pressure lowering and low-dose aspirin in patients with
hypertension: principal results of the Hypertension Optimal
Treatment (HOT) randomised trial. Lancet 351:1755–62, 1998
Tight blood pressure control and risk of macrovascular and
microvascular complications in type 2 diabetes: UKPDS 38 BMJ
317:703–13, 1998
Urine Protein Excretion >1 gram/ 24 hour BP goal
<125/75
Peterson JC, Adler S, Burkart JM, et al. Blood pressure
control, proteinuria, and the progression of renal disease. The
Modification of Diet in Renal Disease Study. Ann Intern Med
123:754– 62, 1995
Angiotensin Receptor Blockers
Renoprotective effect of the angiotensin-receptor antagonist
irbesartan in patients with nephropathy due to type 2 diabetes. N
Engl J Med 345: 851–60, 2001
Effects of losartan on renal and cardiovascular outcomes in
patients with type 2 diabetes and nephropathy. N Engl J Med
345:861–69, 2001
Effects of irbesartan on the development of diabetic nephropathy
in patients with type 2 diabetes. N Engl J Med 345:870–78, 2001
African Americans
Wright JT, Dunn JK, Cutler JA, et al. Outcomes in hypertensive
black and nonblack patients treated with chlorthalidone,
amlodipine, and lisinopril. JAMA 293:1595 -1607, 2 0 05
Wright JT, Bakris G, Greene T, et al. Effect of blood pressure
lowering and antihypertensive drug class on progression of
hypertensive kidney disease: results from the AASK Trial. JAMA
288:2421-31, 2002
Hypertension AlgoritHm for DiAbetes in ADults
2 of 2 – Hypertension Algorithm for Diabetes in Adults – Revised 1/26/12 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Lipid Algorithm For Type 1 and
Type 2 Diabetes Mellitus in Adults
FLP Goals:
LDL-C <100 mg/dL
(<70 with
C
VD
,
C
V
A, o
r
P
VD
)
HD
L-C > 40 mg/d
L
TG <1
50 mg/d
L
Abnormal fasting lipids:
• Initial therapy with TLC & Intensive Glucose Control (with A1c goal < 6%)
•
Eva
luate and treat secondary causes of dyslipidemia: alcohol, estrogen, anabolic steroids, corticosteroids, hypothyroidism, hepatic disease, nephrotic syndrome, chronic renal failure.
•
L
DL
-C i
s the primary target of therapy unless
TG > 4
00 mg/d
L, at w
hich point
TG th
en becomes the primary treatment target.
Isolated low HDL-C (with LDL-C & TG at target)
Optimize
TL
C
, s
moking
cessation, fibrate,
niacin, fish oil or
statin
1
If not at goal
Elevated TG
150-199 Optimize TLC
200-399
Optimize TLC, smoking cessation,
start fibrate, niacin and/or fish oil
> 400
Optimize TLC, smoking cessation,
start fibrate, niacin and/or fish oil
When
TG < 40
0, reassess
L
DL
-C
LDL-C not at goal, follow
elevated
L
DL
-C gui
deline
1
Elevated LDL-C
or
LDL-C at goal with at least one
additional
C
V
ris
k factor present
Start statin, titrate to goal, reinforce TLC
Goal:
L
DL
-C <
100
(<70 if history of
C
VD
, C
V
A, o
r
P
VD
)
If LDL-C remains above goal and/or patient does not tolerate statin,
then add bile acid resin, ezetimibe, niacin or orlistat
Refer to Lipid Specialist
Definitions:
TLC = Therapeutic Lifestyle Changes (refer to TDC Medical
Nut
rition, Weight
Los
s, and
Exe
rcise Algorithms)
Statin = HMG
Co-
A
Red
uctase
Inh
ibitor
TG =
Tri
glycerides
CVD = cardiovascular disease
CVA = cerebrovascular accident
PVD = peripheral vascular disease
Footnotes:
1
If a fibrate is combined with a statin, then fenofibrate is preferred
rather than gemfibrozil due to risk of myositis and rhabdomyolysis.
Determine Fasting Lipid Profile (FLP) yearly
Revised 04/28/11Publication # 45-10777
1 of 2 – Lipid Algorithm For Type 1 and Type 2 Diabetes Mellitus in Adults – Revised 04/28/11 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

HMG CO-A REDUCTASE INHIBITORS LDL-C EQUIVALENCY
IN PATIENTS WITH
HYPERCHO
LESTEROLEMIA*
FLUVASTATIN PRAVASTATIN LOVASTATIN PITAVASTATIN SIMVASTATIN ATORVASTATIN ROSUVASTATIN
EZETIMIBE/
SIMV
ASTATIN
APPROXIMA
TE
%
LDL
↓
20 mg 10 mg 10mg — — — — — 15 –20
40 mg 20 mg 20mg — 5–10mg — — — 21–29
80-
XL 40–80mg 40 mg 1-2 mg 20mg 10mg — — 30–38
— — 80 mg 4 mg 40mg 20mg 5–10mg 10/10 mg 39–47
— — — — 80mg 40mg 20mg 10/20 mg 48–54
— — — — — 80mg 40mg 10/40 mg 55-59
— — — — — — — 10/80 mg >59
* Footnote: This information is not completely based on head to head comparison
Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group.
MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with
diabetes: a randomised placebo-controlled trial. Lancet. 2003 Jun 14;361(9374):2005-16.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol
lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial.
Lancet. 2002 Jul 6;360(9326):7-22.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason
MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS investigators. Primary prevention
of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin
Diabetes Study (CARDS): multicentre randomised placebo-controlled trial.Lancet. 2004 Aug
21-27;364(9435):685-96.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC,
Smith SC Jr, Stone NJ; National Heart, Lung, and Blood Institute; American College of Cardiology
Foundation; American Heart Association.Implications of recent clinical trials for the National
Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004 Jul 13;
110(2):227-39.
Jones P, Kafonek S, Laurora I, Hunninghake D.Comparative dose efficacy study of atorvastatin
versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the
CURVES study) Am J Cardiol. 1998 Mar 1;81(5):582-7.
Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus
gemfibrozil + any statin.Am J Cardiol. 2005 Jan 1;95(1):120-2.
Maeda K, Noguchi Y, Fukui T.The effects of cessation from cigarette smoking on the lipid and
lipoprotein profiles: a meta-analysis. Prev Med. 2003 Oct;37(4):283-90.
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P,
Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C,
Laakso M; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular
events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial.
Lancet. 2005 Nov 26;366(9500):1849-61.
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer
EJ, Schectman G, Wilt TJ, Wittes J.Gemfibrozil for the secondary prevention of coronary heart
disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density
Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999 Aug 5; 341(6):410-8.
Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, Keech A,
Simes J, Peto R, Armitage J, Baigent C.Efficacy of cholesterol-lowering therapy in 18,686 people with
diabetes in 14 randomised trials of statins: a meta-analysis.Lancet. 2008 Jan 12;371(9607):117-25.
Saku K, Zhang B, Noda K. Randomized Head-to-Head Comparison of Pitavastatin, Atorvastatin,
and Rosuvastatin for Safety and Efficacy (Quantity and Quality of LDL). Circ J. 2011 Apr 15. [Epub
ahead of print] PMID: 21498906
Motomura T, Okamoto M, Kitamura T, Yamamoto H, Otsuki M, Asanuma N, Takagi M,
Kurebayashi S, Hashimoto K, Sumitani S, Saito H, Kouhara H, Nshii K, Nakao M, Koga M, Sato
B, Morimoto Y, Kasayama S. Effects of pitavastatin on serum lipids and high sensitivity C- reactive
protein in type 2 diabetic patients. J Atheroscler Thromb. 2009 Oct;16(5):546-52. Epub 2009 Sep 3.
PMID:19729863
REFERENCES
2 of 2 – Lipid Algorithm For Type 1 and Type 2 Diabetes Mellitus in Adults – Revised 04/28/11 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Diabetes treatment algorithms
People with diabetes have a 2 to 4 fold higher risk of dying from cardiovascular disease.
People with diabetes have a complex procoagulant state, which contributes to the increased
risk of atherosclerotic events. Antiplatelet therapy is a simple intervention that can reduce
the risk of events in this high-risk population. NHANES III data shows that 27% of
people with diabetes are eligible for secondary prevention strategies, while an additional
71% had at least one risk factor for atherosclerotic disease. Thus, basically all persons
with diabetes are candidates for antiplatelet therapy, yet only 13% of eligible patients were
currently taking aspirin.1, 2
Recommendations:
1) People with diabetes who are age 30 or above should be offered aspirin therapy if no
contraindications exist to therapy.
2) Dose: 75 to 325mg daily. An enteric-coated product may be used to minimize
gastrointestinal side effects
3) If an aspirin allergy is present, clopidogrel may be recommended (75mg/day) for
secondary prevention. Currently, no primary prevention trials in people with diabetes
have been conducted. In primary prevention patients with multiple risk factors, the risk,
benefit, and cost of clopidrogrel must be considered.
Do not use antiplatelet therapy in people with:
1) Bleeding tendency
2) Anticoagulant therapy
3) Recent
gastrointestinal bleeding
4) Clinically active hepatic disease
5) Patients at risk of Reye’s syndrome
Combination Therapy:
In people with diabetes who have an event on aspirin, aspirin resistance may play a role.3
1) The CURE trial used combination therapy with aspirin 75mg to 325mg and clopidogrel
75mg every day. Though over 22% of the patients enrolled had diabetes, the relative risk
of an event in subjects with diabetes was not reduced significantly by the combination.
2) No benefit has been shown with the addition of warfarin to aspirin therapy 4
Secondary Prevention
1) Anti-platelet Trialists’
Antiplatelet Trialists’ Collaboration: Collaborative overview of randomised trials
of antiplatelet therapy, I: Prevention of death, myocardial infarction, and stroke by
prolonged antiplatelet therapy in various categories of patients. BMJ 308:81-106,1994
Macrovascular Risk Reduction in Diabetes:
Antiplatelet Therapy
1 of 3 – Macrovascular Risk Reduction in Diabetes: Antiplatelet Therapy – Published: 2004
Published 2004 Publication # 45-11942
sUPPlement
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
a. Meta-analysis of 145 prospective controlled trials of antiplatelet therapy
b.
Risk r
eduction of 38±12 vascular events per 1000 diabetics treated (p<0.02)
c.
Placebo
rate of events 22.3%, reduced to 18.5% on doses of 75mg to 325mg a day
2)
Early Treatment of D
iabetic Retinopathy Study (
ETDRS)
ETDRS Investigators: Aspirin effects on mortality and morbidity in patients with
diabetes mellitus. JAMA 268:1292-1300, 1992
a. Mixed group of primary and secondary prevention in 3711 diabetics
b.
Dose:
650mg/day or placebo
c. Results: 9.1% had myocardial infarction (MI) on aspirin vs. 12.3% on placebo
d.
No
increase in retinal bleeding was seen on serial eye exams
3) Hypertension Optimal Treatment (HOT)
Hansson L, Zanchetti A, Carruthers SG, et al: Effects of intensive blood-pressure lowering
and low-dose aspirin in patients with hypertension: Principal results of the Hypertension
Optimal Treatment (HOT) randomised trial. Lancet 351:1755-1762, 1998
a.
Mix
ed primary and secondary prevention trial in hypertensive type 2 diabetics
b.
1501 diabetics
enrolled in study for average of 3.8 years follow-up
c.
Dose:
75mg or placebo
d.
Results:
15% reduction in pooled cardiovascular events (p=0.03), and a 36%
reduction in the risk of MI (p=0.002)
4) Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE)
a.
19185 persons
with recent atherosclerotic event randomized to clopidogrel or aspirin
b.
Dose:
clopidogrel 75mg every day, aspirin 325mg every day
c.
5.32% risk
of ischemic stroke, MI, or vascular death with clopidogrel vs. 5.83% for
aspirin (p=0.043)
d.
Post-hoc
subset analysis of 3866 subjects diagnosed with diabetes by intake
questionnaire from investigator5
e.
Composite outcome endpoint was: vascular death, MI, stroke, or hospitalization for
angina or bleeding event.
f. Event rate was 15.6% vs. 17.7%/ year (p=0.042), for clopidrogrel and aspirin
respectively. No significant difference in individual outcomes.
g.
Would
need to treat approximately 47 individuals with clopidrogrel instead of
aspirin to reduce one event.
5)
Effects of Clopidogrel in A
ddition to Aspirin in Patients with Acute Coronary
Syndromes without S
T-Segment Elevation. The Clopidogr
el in Unstable Angina to
Prevent Recurrent
Events Trial I
nvestigators (CUR
E)
N Eng J Med 345:494-502, 2001
2 of 3 – Macrovascular Risk Reduction in Diabetes: Antiplatelet Therapy – Published: 2004 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
a. 12, 562 subjects who presented to the hospital with an acute coronary syndrome
within 24 hours of symptoms
b.
Giv
en aspirin 75mg to 325mg every day plus one time dose of clopidogrel 300mg,
followed by 75mg every day vs. aspirin alone
c.
Results:
In 2849 subjects who had diabetes, the combination group experienced a
14.2% event rate vs. 16.75% in the aspirin alone group.
d.
Though the
relative risk favored addition of clopidogrel, the reduction was not
significant
6)
Ticlopidine in Micr
oangiopathy of Diabetes (
TIMAD)
TIMAD
Study Group: Ticlopidine treatment reduces the progression of nonproliferative
diabetic retinopathy. Arch Ophthalmol 108:1577-1583, 1990
a.
435 diabetic
with nonproliferative diabetic retinopathy
b.
ticlopidine 250
mg two times a day or placebo
c.
follow
ed up to 3 years
d.
fluorescein
angiograms of eyes done
e.
Reduction
in progression of retinopathy by 67% (p=0.03) in ticlopidine group vs.
placebo
f.
Side effects limit usefulness: 2-3% experience neutropenia, serial CBC’s must be
followed for a minimum of 3 months
Primary Prevention
1) Physician’s Health Study
Steering Committee of the Physicians’ Health Study Research Group: Final report on the
aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 321:129-135, 1989
a. Dose: 325mg every other day or placebo
b.
22, 071 participants followed for approximately 5 years, 533 had diabetes
c. Outcome: myocardial infarction in 11/275 (4.0%) on aspirin vs. 26/258 on placebo
(10.0%). Relative risk = 0.39 (significance not reported)
References:
1.
R
ol
ka
D
B
, Fa
got-
Cam
pagna A,
Nar
ayan KM: Aspirin use among adults with diabetes:
Est
imates from the
Thi
rd
Nat
ional Health and
Nut
rition
Exa
mination
Sur
vey.
Di
abetes
Care
24:197-201, 2001
2.
Am
erican
Di
abetes Association.
Pos
ition
Stat
ement. Aspirin
The
rapy in
Di
abetes.
Di
abetes
Care
. 25:
S78-S79
, 2002;
Di
abetes
Care
. 2004 Jan;27
Sup
pl
1:S72-3.
3. Gum PA, Kottke-Marchan K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 88:230-5, 2001
4.
Fi
ore
L
D
, Eze
kowitz M
D, Rop
hy M
T, et a
l.
Dep
artment of veterans affairs cooperative studies program clinical trial comparing combined warfarin and aspirin with
aspirin alone in survivors of acute myocardial infarction: primary results of the
CHA
M
P s
tudy.
Cir
culation 105:557-563, 2002
5.
C
an
non
C
P
. CAPR
IE
Inv
estigators.
Eff
ectiveness of clopidrogrel versus aspirin in preventing acute myocardial infarction in patients with symptomatic
atherothrombosis (
CAPR
IE
tr
ial). American Journal of
Car
diology 90(7):760-2;2002
3 of 3 – Macrovascular Risk Reduction in Diabetes: Antiplatelet Therapy – Published: 2004 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Insulin Algorithm for Type 1 Diabetes
Mellitus in Children and Adults
1
OR
Split-Mix Insulin Therapies
4
1. Two shots: NPH + Reg or RAI
2:
1 ratio AM; 1:1 ratio
PM
2.
Thr
ee shots: AM:
N
P
H + Reg o
r
RAI
P
M: Reg o
r
RAI
HS: NPH
2/3 TDI ÷ as 2/3 AM NPH + 1/3 as Reg or RAI
1/
3
T
DI
÷ a
s ½
PM Reg o
r
RAI + ½ N
P
H at HS
3.
Two s
hots
Pre
mix
2/
3 AM + 1/3
PM
To
tal Daily Insulin
5
: 0.3-0.5 units/kg/day, and titrate to glycemic targets
Follow A1c Every 3-6 months and Adjust Regimen to Maintain Glycemic Targets
Footnotes
1
Consider referring all type 1 patients to pediatric/adult endocrinologist/comprehensive
diabetes specialty team, and consider continuous glucose monitoring.
If in
sulin pump therapy
is considered-refer to
Cert
ified
Pum
p
Tra
iner.
2
Intensify management if: Absent/stable cardiovascular disease, mild-moderate microvascular
complications, intact hypoglycemia awareness, infrequent hypoglycemic episodes, recently diagnosed
diabetes. Less intensive management if:
Evi
dence of advanced or poorly controlled cardiovascular
and/or microvascular complications, hypoglycemia unawareness, vulnerable patient (ie, impaired
cognition, dementia, fall history).
See “
A1c Goal” treatment strategy for further explanation. A1c
is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA Clinical Practice
Rec
ommendations. Diabetes Care 2009;32(suppl 1):
S19
-20.
3
Modern glucose meters give values corrected to plasma glucose.
4
Most type 1 patients need IIT to attain glycemic targets; IIT may be by SQ multiple injection or by SQ
continuous insulin pump.
5
Dosages may differ in children and adolescents.
6
Twice daily dosing may be required at low basal insulin doses.
7
Strongly recommend referral to Registered/Licensed Dietitian or Certified Diabetes Educator
with experience in diabetes nutrition counseling.
8
Consider decreasing 1 unit for every 30 minutes of vigorous physical activity.
9
IMPORTANT: See package insert for dosing
Intensive Insulin Therapy (IIT)
Physiologic Insulin-1:1 basal:bolus ratio SQ
Basal: Glargine QD or Detemir QD-BID
6,9
Bolus: RAI (or Reg) before each meal: If meal skipped, skip dose.
Premeal insulin dose includes:
1.
I
ns
ulin to cover carbohydrate ingested
7
; 1 unit RAI covers 500/TDI grams
carbohydrate from meal
2.
Ad
ditional insulin to correct for high
SMBG; 1 u
nit
RAI l
owers
PG by a
pproximately
1800/
TD
I
mg
/d
L. (Reg l
owers
PG by ~
1500/
TD
I
)
3.
C
on
sider adjustment for exercise
8
Total Daily Insulin
5
: 0.3-0.5 units/kg/day and titrate to glycemic targets
Pramlintide
1,9
Consider as
adjunct therapy
to insulin in
patients unable
to stabilize
PPG.
ABBREVIATIONS
BASAL: Glargine or
Det
emir
BOLUS (Prandial):
Re
g:
Reg
ular
Ins
ulin (peak action 3-4 hrs)
R
AI:
Rap
id Acting
Ins
ulin = Aspart, Glulisine, or
Lis
pro (peak action 1-1
½ hrs)
PPG:
Pos
t-
Pra
ndial Glucose
SMBG:
Sel
f-monitored blood glucose
3
TDI: Total daily insulin dosage in units
Glycemic Goals
2,3
Individualize goal based on patient
risk factors
A1c
≤6% <7
%
<
8%
FPG ≤1
10
12
0
14
0 mg/d
L
2h P
P
≤1
30
18
0
18
0 mg/d
L
Revised 1/27/10Stock # 45-11649
1 of 1 – Insulin Algorithm for Type 1 Diabetes Mellitus in Children and Adults – Revised 1/27/10
Diabetes treatment algorithms
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Insulin Algorithm for Type 2 Diabetes Mellitus
in Children and Adults
Intensive Insulin Management
10
Basal: Once-daily, either morning or bedtime
(alternative:
N
P
H mo
rning and bedtime)
Bolus: Fast-acting insulin before each meal;
(alternative:
R ma
y be used)
Premeal insulin dose includes:
1.
I
ns
ulin to cover carbohydrate ingested
11
&
2.
Ad
ditional insulin to correct for high
SMBG:
1 e
xtra unit premeal insulin ↓ glucose (mg/d
L)
~1
500/
TD
D
f
or
Reg
ular;
~1800/
TD
D
fo
r Aspart/Glulisine/
Lispr
o
Starting dose
8,9
:
1. 0.3-0.5 units/kg/day (1:1 basal:bolus ratio
SQ)
Or
2
.
If cu
rrent dose >0.5 units/kg/day
Bas
al dose = 80%
Tot
al daily
N
P
H or
8
0% Total long-acting
component of premix
Bol
us dose = 80% of basal dose divided
between 3 meals
Basal insulin: Glargine or
Det
emir
Bolus insulin: Aspart or
Glulisine or
Lispr
o
Fast-acting insulin: Aspart;
Glulisine;
Lispr
o
F: Fast-acting insulin
R:
Reg
ular insulin
FPG: Fasting plasma glucose
PP:
Pos
tprandial plasma
glucose
SMBG:
Self
-monitored blood
glucose
TDD:
Tot
al daily dose of insulin
Treatment Naïve
3
A1c ≥10% or A1c <10% when
considering early insulin initiation
If ke
toacidosis or recent rapid weight
loss, see
Typ
e 1
Dia
betes algorithm
Uncontrolled on
non-insulin therapy:
A1c <1% above goal
• Continue oral agent therapy +/- insulin secretagogue
• Stop GLP-1 agonist prior to insulin therapy
Once-daily Insulin Therapy
Morning – Basal insulin
Bedtime –
Bas
al insulin or
NPH
Bef
ore supper (Evening)
N
P
H + R o
r F 2:1 ratio or
Pre
mix 70/30 or 75/25
Starting dose
9,10
0.1-0.25 units/kg/day or
6-10 units/day if patient is elderly or thin
Achieving Goals
Adjust
Bas
al insulin /
Bed
time
N
P
H ba
sed on F
PG
Ad
just NPH + R or F / Premix based on bedtime
glucose and F
PG
Tit
ration schedule
9
Add 1 unit of insulin each day to
reach glycemic goals OR
If FPG:
>1
80 mg/d
L Ad
d 6 units
If 141–180 mg/dL Add 4 units
If 12
1–140 mg/d
L Ad
d 2 units
If 10
0–120 mg/d
L Ad
d 1 unit
If 80
-99 mg/d
L No ch
ange
If <8
0 mg/d
L
S
ub
tract 2 units
Multi-dose Insulin Therapy (MDI)
10
2 injections
NPH wi
th
R o
r F (ratio 2:1) before AM and evening meal
or
Pre
mix 70/30 or 75/25 before AM and evening meal
3 injections (especially if nocturnal hypoglycemia)
NPH wi
th
R o
r F morning;
R o
r F
7
evening; NPH bedtime or
Pre
mix (as above) morning, noon, evening
Starting dose
8,9
: 0.3-0.5 units/kg/day, divided as follows:
2 injections: 2/3 morning; 1/3 evening
3 injections:
Pre
mix: 1/3 morning; 1/3 noon; 1/3 evening
NPH + R o
r F:
Before
AM Meal
Before
evening meal
At
Bedtime
NPH 40
% of
T
DD
25
% of
T
DD
R
o
r F 20% of
T
DD
15
% of
T
DD
Follow A1c every 3-6 months and Adjust Regimen
to Maintain Glycemic Goals
Glycemic
Goals
Not
Met After
3–6
Months
Glycemic
Goals
Not
Met After
6–12 Weeks
Uncontrolled on
non-insulin therapy:
A1c ≥1% above goal
INITIAL OPTIONS
3,4
• Once-daily Insulin
5
+ oral(s)
• Multi-dose Insulin
6
+/- oral(s)
• Intensive Insulin
Management
6
+/- oral(s)
INITIAL OPTIONS
4
• Once-daily Insulin
5
• Multi-dose Insulin
6
• Intensive Insulin
Management
6
INITIAL OPTIONS
4
• Multi-dose Insulin
6
• Intensive Insulin
Management
6
• Once-daily Insulin
5
Pramlintide
Consider as
adjunct therapy
to insulin in
patients unable
to stabilize
post-prandial
glucose
Glycemic Goals
1,2
Individualize goal based on patient
risk factors
A1c
≤6% <7
%
<
8%
FPG ≤1
10
12
0
14
0 mg/d
L
2h P
P
≤1
30
18
0
18
0 mg/d
L
Footnotes
1
Intensify management if: Absent/stable cardiovascular disease, mild-moderate
microvascular complications, intact hypoglycemia awareness, infrequent
hypoglycemic episodes, recently diagnosed diabetes. Less intensive management
if: Evidence of advanced or poorly controlled cardiovascular and/or microvascular
complications, hypoglycemia unawareness, vulnerable patient (ie, impaired cognition,
dementia, fall history). SEE “A1c Goal” treatment strategy for further explanation. A1c
is referenced to a non-diabetic range of 4-6% using a DCCTbased assay. ADA Clinical
Practice Recommendations Diabetes Care 2009;32(suppl 1):S19-20.
2
Current glucose meters give values corrected to plasma glucose.
3
May also begin combination oral agent therapy. See Glycemic Control Algorithm for
Type 2 Diabetes Mellitus in Children and Adults.
4
Combining metformin with insulin therapy has been shown to result in less weight
gain and better glycemic control with lower insulin requirements.
5
Continue combination oral agent therapy + sulfonylurea.
6
Continue metformin (+ 3rd oral agent); probably discontinue sulfonylurea.
7
Fast-acting insulin is given with the start of each meal. Regular insulin to be given
30-60 minutes before meals.
8
Dosage may differ in children and adolescents; consider referral to pediatric
endocrinologist/comprehensive diabetes specialty team.
9
Start lower and increase slower for thin/elderly/complicated patients.
10
Consider referral to pediatric/adult endocrinologist/diabetes specialty team
(option – insulin pump, Pramlintide).
11
Typical “carb” bolus = 1 unit bolus insulin covers 500/TDI x g carbohydrate from meal
(~10-15 gm); strongly recommend referral to Registered/Licensed Dietitian or
Certified Diabetes Educator with experience in diabetes nutrition counseling
(see Worksheet D).
Revised 10/28/10Stock # 45-11647
1 of 6 – Insulin Algorithm for Type 2 Diabetes Mellitus in Children and Adults – Revised 10/28/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Initiation of Once Daily Insulin Therapy for Type 2 Diabetes Mellitus
in Children and Adults
Treatment Naïve
3
:
A1c ≥10% or A1c <10% when
considering early insulin initiation
If ketoacidosis or recent rapid weight loss,
see
Typ
e 1
Dia
betes algorithm
Oral Agent Failure;
A1c above target
Initiate Insulin Therapy with daily Glargine or Detemir or bedtime NPH
5,6
Beginning Dosage: 10 units or 0.1–0.25 units/Kg
Suggested Titration Schedule – Adjust Every 2-3 Days
If FPG:
>180 mg/dL Add 6 units
If 14
1–180 mg/d
L Ad
d 4 units
OR Ad
d 1unit insulin each day until
If 12
1–140 mg/d
L Ad
d 2 units
fa
sting
SMBG is a
t goal
If 10
0–120 mg/d
L Ad
d 1 unit
If 80
-99 mg/d
L No ch
ange
If <80 mg/dL Subtract 2 units
If A1c remains >A1c goal over 3 months, discontinue oral secretagogue, continue oral insulin
sensitizer(s) and initiate multi-dose insulin or intensive insulin therapy
1
or consult an endocrinologist
Footnotes
1
For the complete approach to insulin initiation in Type 2 Diabetes Mellitus, see Insulin Algorithm
for
Typ
e 2
Dia
betes Mellitus in
Chi
ldren and Adults.
2
Intensify management if: Absent/stable cardiovascular disease, mild-moderate microvascular
complications, intact hypoglycemia awareness, infrequent hypoglycemic episodes, recently
diagnosed diabetes. Less intensive management if:
Ev
idence of advanced or poorly controlled
cardiovascular and/or microvascular complications, hypoglycemia unawareness, vulnerable
patient (ie, impaired cognition, dementia, fall history).
See “
A1c Goal” treatment strategy for further
explanation. A1c is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA
Cli
nical
Prac
tice
Rec
ommendations. Diabetes Care 2009;32(suppl 1):
S19
-20.
3
Current glucose meters give values corrected to plasma glucose.
4
Usually with an insulin secretagogue (sulfonylurea, repaglinide or nateglinide) and sensitizer
(metformin or thiazolidinedione).
See G
lycemic
Con
trol Algorithm.
5
The pharmacokinetic profile of NPH compared to that of glargine or detemir is less predictable,
therefore can result in blood sugar variations and increased nocturnal hypoglycemia.
Cos
t of
glargine or detemir is 1.5-2 times that of
N
P
H. Lis
pro 75/25 or Aspart 70/30 can be considered at
pre-supper adjusting dosage according to H
S a
nd fasting
SMBG.
6
IMPORTANT: See package insert for dosing.
7
If daytime hypoglycemia develops, contact healthcare professional.
Abbreviations:
FPG:
Fa
sting plasma glucose
SMBG: Self-monitored blood glucose
PP:
Pos
tprandial plasma glucose
Glycemic Goals
2,3
Individualize goal based on patient
risk factors
A1c
≤6% <7
%
<
8%
FPG ≤1
10
12
0
14
0 mg/d
L
2h P
P
≤1
30
18
0
18
0 mg/d
L
Revised 10/28/10Stock # 45-11647
2 of 6 – Initiation of Once Daily Insulin Therapy for Type 2 Diabetes Mellitus in Children and Adults – Revised 10/28/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Note: “Analog” = Rapid Acting (Bolus) Analog insulin throughout this document.
A. Conversion from once-daily insulin to intensive/physiologic insulin replacement:
Oral therapy failure: Once-daily glargine was added to the oral regimen and titrated to 30 units per day. How do you add analog insulin if the patient
reports the following SMBG values?
FPG
2-HR PP
BRKFT
2-HR PP
LUNCH
2-HR PP
DINNER
Case 1 105 140 140 240
Case 2 105 140 190 240
Case 3 105 190 240 240
Case 1
a. Continue the oral agents (± sulfonylurea) and 30 units glargine or detemir (or NPH)
b. There are 2 approaches for adding analog (RAI) 10-15 minutes before a meal:
#1
Arbitrary
start:
5 units
Titrate: Add 2 units every 2 days to reach 2-hr pp goal
#2 Carb-counting 1 unit/50 mg/dL over 2-hr pp goal
PLUS
1 unit/15 grams carbohydrate
Titrate: Add 1 unit/50 mg/dL >2-hr pp goal every 2 days
Cases 2 and 3
As above, but add and titrate analog before each meal where the postprandial glucose is above goal. Also, see part D below for more information on how
to optimize the use of analog insulin. Re-evaluate each week to be certain that about half of the total daily dose is basal and half is bolus insulin.
B. Conversion from once-daily premix to intensive/physiologic insulin replacement:
Oral therapy failure: Once-daily 70/30 premixed insulin was added and titrated to 30 units per day. The fasting glucose is at goal, but daytime
control is poor. How do you convert to physiologic insulin therapy?
Worksheet: Advancing to Intensive/Physiologic Basal:
Bolus Insulin Therapy
Revised 1/27/10Stock # 45-11647
3 of 6 – Worksheet: Advancing to Intensive/Physiologic Basal: Bolus Insulin Therapy – Revised 1/27/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

a. Basal insulin dose: The first step in the conversion is based on the total dose of intermediate-acting insulin. In this case, the person is taking 21
units of NPH or aspart-protamine insulin (70% x 30 units=21 units). So, give 21 units basal glargine (use “unit-for-unit” conversion for once-
daily intermediate regimens). Remember, do not stop oral agents (+ sulfonylurea) at this time.
b.
Bolus insulin dose:
There are several ways to start the analog.
i.
See
Case 1 (Arbitrary start or Carb-counting)
ii. Begin with the previous dose of fast-acting insulin, divide it before meals and titrate every 2 days. In this case, the person was using
30 units of 70/30 or about 9 units of fast-acting insulin (30% x 30 units=9 units). So give 3 units of analog before each meal and
titrate every 2 days as per Case 1.
C. Conversion from twice-daily premix to intensive/physiologic insulin replacement:
Oral therapy failure in an 80 kg person: 70/30 premixed insulin was started and advanced to 60 units per day: 40 units before breakfast and 20 units
before dinner. The fasting glucose was at goal, but wide glycemic excursions occurred at other times during the day and night. How do you convert
this person to physiologic insulin therapy? There are several approaches. Use which ever method you want.
a.
Star
t over and begin insulin at 0.5 units/kg. Give half as basal insulin and half as analog, divided before meals. In this case, the starting
dose would be 40 units per day. Start giving 20 units glargine each morning and about 7 units analog before each meal. Titrate the basal
and bolus insulins every 2 days to fasting and 2-hr postprandial goals.
b.
Conversion
based on current insulin usage:
Basal dose:
The first step in the conversion is based on the 80% of the total dose of intermediate-acting insulin. In this case, the
person is taking 42 units of NPH or aspart-protamine insulin (70% x 60 units = 42 units). When a person is taking multiple doses of
intermediate-acting insulin, we give only 80% as glargine. So, give 34 units basal glargine (80% x 42=~34). Remember, do not stop oral
agents (+ sulfonylurea) at this time.
Bolus insulin dose: There are several ways to start the analog.
i. See Case 1 (Arbitrary start or Carb-counting)
ii. Begin with the previous dose of fast-acting insulin, divide it before meals and titrate every 2 days. In this case, the person was using
60 units of 70/30 or 18 units of fast-acting insulin (30% x 60 units = 18 units). So, give 6 units of analog before each meal and
titrate every 2 days as per Case 1.
c. The “80%-80%”rule: Similar to the above method, but yields an ideal ratio of basal:bolus insulin in one step. The dose of basal glargine
will be 80% of the total intermediate insulin, and the analog will be 80% of the glargine dose, divided before meals.
4 of 6 – Worksheet: Advancing to Intensive/Physiologic Basal: Bolus Insulin Therapy – Revised 1/27/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Basal dose: = 80% of total intermediate insulin
=
80% x
42 units (70% x 60 = 42)
=
34 units
glargine
Analog dose:
= 80% of
the glargine dose, divided TID
= 80% x
34 units = 27 units
= 27
units, divided TID = 9 units
= 9 u
nits aspart, glulisine or lispro before meals
Note:
To
tal dose of insulin is conserved and an ideal ratio between basal and bolus will always result with the “80%-80%” method.
D. Optimizing analog insulin use
Tight control of blood glucose requires that the patient participates in the management of their diabetes. This includes monitoring their blood
glucose and learning to count carbohydrates or “carb count.” The following material explains how to calculate the dose of analog required to cover
a meal and how to add extra analog to correct a hyperglycemic event.
a. Determining the dose of analog insulin to use before a meal
The “Rule of 500” is used to determine how many grams of carbohydrate 1 unit of analog insulin will cover. When this number is
known, then the person can easily give the correct dose of analog by simply counting the grams of carbohydrate they intend to eat at the
meal.
Specifically
, 500 divided by the total daily insulin dose (500/TDI) yields the number of grams of carbohydrate that 1 unit of analog will
cover. For example, if a person has established that they require about 50 units of insulin per day, then it follows that 1 unit of analog will
cover 10 grams of carbohydrate (500/50 = 10). If the person carb counts 140 grams in the dinner meal, then the dose of analog will be 14
units given 10 minutes before eating.
b.
Correcting for hyperglycemia
The “Rule of 1800”
is used to determine how much insulin to use to bring a high glucose reading back to goal. Even with tight control,
hyperglycemia occurs and people need to be able to correct this situation.
Specifically
, 1800 divided by the total daily insulin dose yields a value indicating how much 1 unit of analog insulin will lower the blood
glucose. Thus, if a person uses 90 units of insulin per day, then 1 unit of analog will reduce the blood glucose by 20 mg/dL
(1800/90 = 20).
This augment dose of insulin can be used b
y itself to correct hyperglycemia, or added to the bolus dose if glucose is
high before a meal.
5 of 6 – Worksheet: Advancing to Intensive/Physiologic Basal: Bolus Insulin Therapy – Revised 1/27/10 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

References
1. Riddle MC, Rosenstock J, Gerich J. Diabetes Care. 2003; 26:3080-3086.
2. Sp
ellman CW, Renda SM, Davis SN. Realizing the Potential of Insulin Therapy in Type 2 Diabetes: A Case Presentation-Based Monograph. presented at the American College of
Osteopathic Internists 64th Annual Convention; Chicago, IL (September 30, 2004).
3.
ww
w.texasdiabetescouncil.org.
6 of 6 – Worksheet: Advancing to Intensive/Physiologic Basal: Bolus Insulin Therapy – Revised 1/27/10
Reviews/Important Articles
• AbrairaC,ColwellJ,NuttallF,etal.CardiovasculareventsandcorrelatesintheVeterans
Affairs D
iabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and
Complications in Type II Diabetes. Arch Intern Med. 1997;157(2):181-8.
• Anonymous.
Intensiv
e
blood-glucosecontrolwithsulphonylureasorinsulincomparedwith
conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK
Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-53.
• DeWitt
DE,DugdaleDC.Usingnewinsulinstrategiesintheoutpatienttreatmentofdiabetes:
clinical applications.
JAMA. 2003;289(17):2265-9.
• DeWittDE,HirschIB.Outpatientinsulintherapyintype1andtype2diabetesmellitus:scientific
revie
w. JAMA. 2003;289(17):2254-64.
• Implementation
ConferenceforACEOutpatientDiabetesMellitusConsensusConference
Recommendations: P
osition Statement, February 2, 2005. Available online at http://www.aace.com/
pub/odimplementation/PositionStatement.pdf. Accessed on May 2, 2005.
• HirschIB.Insulinanalogues.N Engl J Med. 2005;352(2):174-83.
Once Daily Insulin
Morning vs. Bedtime NPH
• GroopLC,WidenE,EkstrandA,etal.MorningorbedtimeNPHinsulincombinedwith
sulfonylurea in treatment of NIDDM. Diabetes Care. 1992;15(7):831-4.
Morning vs. Bedtime Glargine
• FritscheA,SchweitzerMA,HaringHU.Glimepiridecombinedwithmorninginsulinglargine,
bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2
diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138(12):952-9.
NPH vs. Glargine
• RiddleMC,RosenstockJ,GerichJ.Thetreat-to-targettrial:randomizedadditionofglargineor
human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-6.
Once Daily vs. Twice Daily Regimen
• RaskinP,AllenE,HollanderP,etal.Initiatinginsulintherapyintype2diabetes:acomparisonof
biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260-5.
Multiple Dose Insulin Regimens (2-shot Regimens)
NPH/Regular vs. NPH/ short acting analogue therapy
• VignatiL,AndersonJHJr,IversenPW.EfficacyofinsulinlisproincombinationwithNPHhuman
insulin twice
per day in patients with insulin-dependent or noninsulin-dependent diabetes mellitus.
Multicenter Insulin Lispro Study Group. Clin Ther. 1997;19(6):1408-21.
70% NPH/ 30% Regular vs. Humalog Mix 75/25™ or Novolog Mix 70/30™
• Roach
P,YueL,AroraV.Impr
oved
postprandialglycemiccontrolduringtreatmentwithHumalog
Mix25,
a novel protamine-based insulin lispro formulation. Humalog Mix25 Study Group. Diabetes
Care. 1999;22(8):1258-61.
• Boehm
BO,HomePD,Behr
end
C,etal.Pr
emixed
insulinaspart30vs.premix
ed
human
insulin 30/70
twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med.
2002;19(5):393-9.
Multiple Dose Insulin Regimens (3-shot Regimens)
• OhkuboY,KishikawaH,ArakiE,etal.Intensiveinsulintherapypreventstheprogressionof
di
abetic microvascular complications in Japanese patients with non-insulin-dependent diabetes
mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103-17.
Intensive Insulin Therapy
• OhkuboY,KishikawaH,ArakiE,etal.Intensiveinsulintherapypreventstheprogressionofdiabetic
microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a
randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103-17.
• SaudekCD,DuckworthWC,Giobbie-HurderA,etal.Implantableinsulinpumpvsmultiple-dose
insulin for
non-insulin-dependent diabetes mellitus: a randomized clinical trial. Department of
Veterans Affairs Implantable Insulin Pump Study Group. JAMA. 1996;276(16):1322-7.
• Raskin
P,BodeBW
,
Mar
ks
JB,etal.Continuoussubcutaneousinsulininfusionandmultipledaily
injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week
study. Diabetes Care. 2003;26(9):2598-603.
• Bretzel
RG,ArnoldsS,MeddingJ,etal.Adirectefficacyandsafetycomparisonofinsulinaspart,
human soluble insulin, and human premix insulin (70/30) in patients with type 2 diabetes. Diabetes
Care. 2004;27(5):1023-7.
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

This algorithm is not intended to be used for those individuals with Type 1 diabetes, diabetic
ketoacidosis or hyperglycemic hyperosmolar states.
TARGET RANGE FOR GLYCEMIC CONTROL:
80–140 mg/dL (Generally 110 mg/dL)
1. Standard drip 100 units/100 mL 0.9% NaCl.
Appr
oved IV insulins include Regular, aspart and glulisine
2.
Star
t IV insulin therapy when glucose is above target range. Insulin infusions should be
discontinued when
a. Patient
has no history of diabetes and is receiving <1 Unit/hour
b. Patient
receives 1st dose of SC basal + bridging dose of fast analog or R (see #10)
3. Bolus dose and Initial Infusion rate: Divide initial glucose level by 100, then round to
nearest 0.5 units for bolus AND initial infusion rate
Ex
amples:
1) In
itial glucose=326 mg/dL: 326÷100=3.26, round to 3.5: IV bolus
3.5 units + start infusion @ 3.5 units/hour
2) In
itial glucose=174 mg/dL: 174÷100=1.74, round to 1.5: IV bolus 1.5
units + start infusion @1.5 units/hour
4. Intravenous Fluids
u
Most patients will need 5–10 g glucose per hour D5W or D5W½NS at 100–200
mL/hour or equivalent (TPN, enteral feeding, etc.)
5. Adjusting the Infusion:
u
Algorithm 1: Start here for most patients.
u
Algorithm 2: For patients not controlled with Algorithm 1, or start here if s/p CABG,
solid organ or islet cell transplant, receiving glucocorticoids etc. or patient with diabetes
receiving >80 units/day of insulin as an outpatient.
u
Algorithm 3: For patients not controlled on Algorithm 2. NO PATIENT
STARTS HERE without authorization from the endocrine service.
u
Algorithm 4: For patients not controlled on Algorithm 3.
NO PATIENT STARTS HERE
IV Insulin Infusion Protocol
for Critically-Ill Adult Patients in the ICU Setting
Diabetes treatment algorithms
Revised 10/25/07Publication # 45-12063
1 of 4 – IV Insulin Infusion Protocol for Critically-Ill Adult Patients in the ICU Setting – Revised 10/25/07 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Algorithm 1 Algorithm 2 Algorithm 3 Algorithm 4
Glucose units/h Glucose units/h Glucose units/h Glucose units/h
<60 = Hypoglycemia (See #8 for treatment)
<70 Off <70 Off <70 Off <70 Off
70–109 0.2 70–109 0.5 70–109 1 70–109 1.5
110–119 0.5 110–119 1 110–119 2 110–119 3
120–149 1 120–149 1.5 120–149 3 120–149 5
150–179 1.5 150–179 2 150–179 4 150–179 7
180–209 2 180–209 3 180–209 5 180–209 9
210–239 2 210–239 4 210–239 6 210–239 12
240–269 3 240–269 5 240–269 8 240–269 16
270–299 3 270–299 6 270–299 10 270–299 20
300–329 4 300–329 7 300–329 12 300–329 24
330–359 4 330–359 8 330–359 14 330–359 28
>360 6 >360 12 >360 16 >360 32
6. Moving from Algorithm to Algorithm:
u
Moving Up: When glucose remains outside the target range after titrating insulin
u
Moving Down: When glucose is <70 mg/dL x 2 or decreases >60 mg/dl in 1 hour
7. Patient Monitoring:
u
Hourly venous (lab) determinations until glucose <450 mg/dL; then capillary glucose
(finger sticks) q 1hour until glucose is within goal x 4 hours; then every 2 hours x 4 hours;
If stable, decrease monitoring to every 4 hours
u
Hourly monitoring indicated for critically ill patients even if the glucose is stable
u
In hypotensive patients (BP <80/60), capillary glucose values may be inaccurate. Obtain
venous blood for glucose determinations
u
If any of the following occur, temporarily resume hourly glucose monitoring, until glucose
is again stable (2–3 consecutive values within target range):
Any change in insulin infusion rate
Significant
changes in clinical condition
Star
ting or stopping pressor or steroid therapy
Starting or stopping dialysis
Starting, stopping or changing rates of TPN, PPN or tube feedings
8. Treatment of Hypoglycemia (Glucose <60 mg/dL)
u
Discontinue insulin drip AND
u
Give D50W IV Glucose 40–60 mg/dL 12.5 g (1/2 amp)
Glucose <40 mg/dL 25.0 g (1 amp)
u
Recheck glucose every 15–30 minutes and repeat D50W IV as above. Restart insulin drip,
one algorithm lower, when glucose >80 mg/dL x 2
Diabetes treatment algorithms
2 of 4 – IV Insulin Infusion Protocol for Critically-Ill Adult Patients in the ICU Setting – Revised 10/25/07 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

9. Notify the physician:
u
For patients not responding to Algorithm 1 or 2.
u
For hypoglycemia which has not resolved after administration of D50W IV and
discontinuation of the insulin drip
10. Transition from IV insulin to SC insulin: “Basal-Analog” Method
a.
Ca
lculate Total Daily Dose (TDD) for subcutaneous insulin
TDD = Infusion rate/h x 20h
b.
Fir
st dose SQ insulin includes [basal insulin + bridging dose aspart, glulisine,
lispro or R] x 1
1.
If patient will begin eating give:
•
Ha
lf
TD
D
asba
sal
gl
argine,
de
temir*
orNP
H*
Pl
us
•
Bri
dging
in
sulin**
@10
%
ofba
sal
in
sulin
do
se
•
St
op
IVin
sulin
•
Co
ntinue
pr
imary
I.
V.
2.
If patient will continue NPO, TPN or tube feeding give:
•
Al
l
TD
D
asba
sal
gl
argine,
de
temir*
orNP
H*
Pl
us
•Bridginginsulin**@5%ofbasalinsulindose
•
St
op
IVin
sulin
an
d
co
ntinue
pr
imary
I.
V.
c.
Pr
oceed to “Inpatient Management of Insulin in the Non-Critical Care Setting”
algorithm
fo
r
ma
nagement
ofda
ily
ba
sal
in
sulin,
pr
andial
+su
pplemental
in
sulin**
* Noevidence-baseddataoninpatienttransitionfromI.V.insulintodetemir.Ifdetemirisselected,expecttouseat
least 25% greater dose than glargine. If the dose of detemir is <0.6 units/Kg, use half bid. If NPH is used as a basal
insulin the dose is 2/3 of the TDD (whether or not the patient is eating) and is distributed bid as 2/3 A.M. and 1/3
H.S. or may be divided equally and given q 6h.
** R
(r
egular
in
sulin)
isno
t
pr
eferred
asabr
idging
orpr
andial
in
sulin
Diabetes treatment algorithms
3 of 4 – IV Insulin Infusion Protocol for Critically-Ill Adult Patients in the ICU Setting – Revised 10/25/07 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

REFERENCES:
1. Ga
rber AJ, Moghissi ES, Bransome ED Jr., et al; American College of Endocrinology Task Force on
Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient
diabetes and metabolic control. Endocr Pract. 2004;10 (Suppl 2):4–9.
2.
Bo
de BW, Braithwaite SS, Steed RD, et al. Intravenous insulin infusion therapy: indications, methods, and
transition to subcutaneous insulin therapy. Endocr Pract. 2004;10 (Suppl 2):71–80.
3.
Go
ldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion
protocol in a medical intensive care unit. Diabetes Care. 2004;27(2):461–7.
4.
Vo
ra AC, Saleem TM, Polomano RC, et al. Improved perioperative glycemic control by continuous
insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.
Endocr Pract. 2004;10(2):112–8.
5.
Ch
audhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute
ST-segment-elevation myocardial infarction. Circulation. 2004;109(7):849–54.
6.
Tr
ence DL, Kelly JL, Hirsch IB. The rationale and management of hyperglycemia for in-patients with
cardiovascular disease: time for change. J Clin Endocrinol Metab. 2003;88(6):2430–7.
7.
Li
en L, Spratt S, Woods Z, et al. A new intravenous insulin nomogram in intensive care units improves
management of persistent hyperglycemia (Abstract). Diabetes. 2003;52 (Suppl 1):A125.
8.
Pr
eiser JC, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin
Nutr Metab Care. 2002;5(5):533–7.
9.
Ma
rkovitz LJ, Wiechmann RJ, Harris N, et al. Description and evaluation of a glycemic management
protocol for patients with diabetes undergoing heart surgery. Endocr Pract. 2002;8(1):10–8.
10.
Va
n den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N
Engl J Med. 2001;345(19):1359–67.
11.
Hi
rsch IB. Insulin therapy for diabetes: is the future now? Clin Diabetes. 2001;19:146–7.
12.
Fu
rnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the
incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann
Thorac Surg. 1999;67(2):352–60.
13.
Ma
lmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by
subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study):
effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65.
14.
Wo
o J, Lam CW, Kay R, et al. The influence of hyperglycemia and diabetes mellitus on immediate and
3-month morbidity and mortality after acute stroke. Arch Neurol. 1990; 47(11):1174–77.
15.
Wa
tts NB, Gebhart SS, Clark RV, et al. Postoperative management of diabetes mellitus: steady-state glucose
control with bedside algorithm for insulin adjustment. Diabetes Care. 1987;10(6):772–8.
16.
Pi
ttas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of
randomized controlled trials. Arch Intern Med. 2004;164(18):2005-11.
17.
De
santis AJ, Schmeltz LR, Schmidt K et.al. Inpatient management of hyperglycemia: The northwestern
experience. Endocrine Practice. 2006;12(5):491-505.
18.
Do
naldson S, Villanuueva G, Rondinelli L, Baldwin D. Rush university guidelines and protocols for the
management of hyperglycemia in hospitalized patients. Elimination of the sliding scale and improvement
of glycemic control throughout the hospital. The Diabetes Educator. 2006;32(6):954-962.
19.
Hi
rsch IB. An endocrinologist’s view on the practical use of insulin. Insulin. 2006;1(Suppl A):S18-23.
20.
No
vo Nordisk detemir monograft, 2005. Studies 1337,1530, NNTTT, 1373.
Diabetes treatment algorithms
4 of 4 – IV Insulin Infusion Protocol for Critically-Ill Adult Patients in the ICU Setting – Revised 10/25/07 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
(Not intended for use in patients with type 1 diabetes, DKA or hyperglycemic hyperosmolar states)
1) Start an IV Insulin Flow Sheet and keep record at bedside
2) Start IV:
_____
D5W at 100ml/h
_____
D5W½NS at
_______
ml/h
_____
Other:
_______________________________________________
3) Mix standard insulin drip:
u
100 units Regular, aspart or glulisine insulin in 100 cc NS (1 unit insulin /cc) (Circle one)
4) Give initial insulin bolus:
u
Bolus units of I.V. insulin = Glucose ÷ 100 (e.g. if glucose = 240 mg/dL, give 2.5 units)
5) Start insulin infusion:
u
Initial infusion rate of insulin units/h = Glucose ÷ 100 ( e.g. if glucose=240, begin 2.5 units/h)
6) Target range for glucose:
u
Low Target (circle one) High Target (circle one)
70 100 or
________
mg/dL 110 120 140 or
________
mg/dL
7) Monitor capillary (finger stick) glucose every hour:
u
Obtain lab glucose if finger stick BG is <40 or >400 mg/dL
u
Change frequency of glucose monitoring to:
_______________________________
8) Adjust insulin infusion rate each hour after initial insulin bolus and infusion
Start on Algorithm 1 (No patient begins on Algorithm 3 or 4 without endocrine service authorization)
Start on Algorithm 2 (s/p CABG, transplant, glucocorticoids or >80 units/d insulin outpatient)
u
Move up or down on the same algorithm each hour if glucose remains outside the target range
u
Advance to the next algorithm (i.e. 1g2 etc.) if outside target range at highest infusion rate
u
Treat for hypoglycemia is glucose <60 mg/dL (see # 9)
u
Decrease 1 algorithm (i.e. 3g2 etc.) if glucose 60-69 mg/dL x 2 or decreases >60 mg/dL in 1 hour
Algorithm 1 Algorithm 2 Algorithm 3 Algorithm 4
BG units/h BG units/h BG units/h BG units/h
<60 = Hypoglycemia (See #9 for treatment)
<70 Off <70 Off <70 Off <70 Off
70–109 0.2 70–109 0.5 70–109 1 70–109 1.5
110–119 0.5 110–119 1 110–119 2 110–119 3
120–149 1 120–149 1.5 120–149 3 120–149 5
150–179 1.5 150–179 2 150–179 4 150–179 7
180–209 2 180–209 3 180–209 5 180–209 9
210–239 2 210–239 4 210–239 6 210–239 12
240–269 3 240–269 5 240–269 8 240–269 16
270–299 3 270–299 6 270–299 10 270–299 20
300–329 4 300–329 7 300–329 12 300–329 24
330–359 4 330–359 8 330–359 14 330–359 28
>360 6 >360 12 >360 16 >360 32
ICU Insulin Orders –
IV Insulin Infusion Protocol
Diabetes treatment algorithms
Revised 2/21/08Publication # E45-12614
1 of 2– ICU Insulin Orders – IV Insulin Infusion Protocol – Revised 2/21/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

9) Treat for hypoglycemia if glucose <60 mg/dL or _______________ mg/dL.
u
Glucose 40-59 mg/dL: Give ½ ampule (12.5 grams glucose) D50W by slow IV push over 30 seconds.
u
Glucose <40 mg/dL: Give 1 ampule D50W (25 grams glucose) by slow IV push over 30 seconds
u
Decrease insulin drip rate by moving down 1 algorithm (i.e. from Algo 3 to Algo 2, etc.)
u
Recheck glucose in 15 minutes and repeat D50W, as above, if necessary
10) Call
Endocrine S
ervice if:
u
Other physicians make changes to subcutaneous or IV insulin regimen
u
TPN, steroids or feedings are started, stopped or changed
u
Other physicians turn off the insulin drip for any reason
u
Patient does not respond to above pathways for glycemic control
11)
Transition from IV insulin to SC insulin:
Proceed to the Insulin Transition Pathway
Physician:
________________________________
Time:
____________
Date:
_____________
2 of 2– ICU Insulin Orders – IV Insulin Infusion Protocol – Revised 2/21/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Orders for Adults with DKA and
Hyperglycemic Hyperosmolar State (HHS)
These orders may be initiated in the Emergency Department
DKA: Moderate ketonemia, arterial pH <7.3, serum glucose >250 mg/dL, serum bicarbonate <18 mEq/L
HHS: Ser
um glucose >600 mg/d
L, mi
nimal ketonemia or ketonuria, serum bicarbonate >15 m
Eq/L, pH ≥
7.3
Admit Date: Time: Location: Attending
Diagn
osis
Drug allergies or adverse
reactions
No kn
own drug allergies
List
:
Monitor and Record
1. Vital signs & I&O every hour until stable, then every 2 hours x 24 hours
Ins
ert Foley if no urine output within first hour or within
hour
s
2.
ST
AT f
ingerstick (capillary) blood glucose
(
Use ve
nous or arterial draw if glucose >450 or <45 mg/d
L o
r
S
BP
<
60 mmHg)
Neuro checks every 2 hours (maintain seizure precautions) x 24 hours
Die
t
NPO Ice Chips
Other
:
Activity
Bed r
est
Bat
hroom privileges with assistance
Other
:
Admission lab
S
T
AT M
etabolic
Pro
file (Glucose,
B
UN
, Cre
atinine,
Na, K
,
Cl, HCO
2
, Ca)
Ser
um ketones
Ser
um
P
O
4
, Mg Arterial blood gas CBC with diff.
Blo
od cultures x 2
Uri
ne
C&S A1C
T
S
H
ß
-hydroxybutyrate
Ser
um osmolarity (measured)
Record acidosis-ketosis gap (AKG = arterial pH – plasma ß-hydroxybutyrate. AKG >3 may indicate drug abuse
5
)
Other
:
Additional labs & studies
K and HCO
3
every hour(s). Call results to physician (hourly monitoring is recommended)
Metabolic profile every 4 hours x 24 hours.
Call r
esults to physician
Ca, P
O
4
, Mg every hours x 24 hours. Call results to physician
Rec
ord anion gap AG = (
Na) – (Cl + HCO3)
EKG
Che
st X-ray
Por
table chest X-ray
Culture and sensitivity of:
Other:
Initial IV fluids
Run IV at ml per hour for hours (Adjust for fluid volume already given in ER)
Use 0.9% NaCl if corrected sodium is low (less than mEq/L)
0.45% NaCl if corrected serum sodium is normal or elevated
(Corrected sodium: Add 1.6 mEq to Na lab value for each 100 mg/dL glucose greater than 100 mg/dL)
Other
:
Mi
x standard insulin drip Discontinue all previous insulin orders
Mix 100 units Regular insulin in 100 mL NS
Other: Mix units of insulin in mL NS
Give initial IV insulin bolus
Bolus units Regular insulin IV (recommend 10-15 units Regular insulin IV)
Other: Bolus units of insulin in mL NS
Start insulin infusion
Start insulin infusion at units per hour
Recommend infusion rate is calculated as: Glucose mg/dL ÷ 100 (Ex: Glucose=350 Start 3.5 units/h)
1
of 3– Orders for Adults with DKA and Hyperglycemic Hyperosmolar State (HHS) – Approved 7/31/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 7/31/08

Target range for glucose Rate of glucose reduction not to exceed 100 mg/dL per hour
DKA: 100 to 130 mg/d
L Other
HH
S:
Low t
arget:
High
target:
Monitor glucose every hour Obtain lab glucose if fingerstick blood glucose is >450 or <45 mg/dL or SBP <60 mmHg
Chan
ge frequency of glucose monitoring to:
Adjust insulin infusion rate
Not
e:
No pa
tient begins on Algorithm 3 or 4 without endocrine service authorization
Sta
rt on Algorithm 1
Start on Algorithm 2 (Consider if s/p CABG, transplant, glucocorticoid therapy, >80 U/d insulin)
• Move up or down on the same algorithm each hour if glucose remains outside target range
• Ad
vance one algorithm column (i.e. 12, etc.) if glucose is outside the target range at highest infusion rate
• Tr
eat for hypoglycemia if glucose is <60 mg/dL
• De
crease one algorithm column (i.e. 21, etc.) if glucose is 60-69 mg/d
L x 2 o
r decreases >60 mg/d
L i
n 1 hour
Algorithm 1 Algorithm 2 Algorithm 3 Algorithm 4
BG un
its/h
BG un
its/h
BG un
its/h
BG un
its/h
<60 = Hypoglycemia
<70
Of
f <70
Of
f <70
Of
f <70
Of
f
70–109 0.2 70–109 0.5 70–109 1 70–109 1.5
110 –119 0.5 110–119 1 110 –119 2 110 –119 3
120–149 1 120–149 1.5 120–149 3 120–149 5
150 –179 1.5 150–179 2 150 –179 4 150–179 7
180–209 2 180–209 3 180–209 5 180 –209 9
210–239 2 210–239 4 210–239 6 210–239 12
240–269 3 240–269 5 240–269 8 240–269 16
270–299 3 270–299 6 270–299 10 270–299 20
300–329 4 300–329 7 300–329 12 300–329 24
330–359 4 330–359 8 330–359 14 330–359 28
>360 6 >360 12 >360 16 >360 32
Treat hypoglycemia 1. • Glucose <40 mg/dL: Give 1 ampule D50W (25 grams) by slow IV push over 30 seconds
• De
crease insulin infusion by moving down 1 algorithm (i.e. 21, etc.)
• Re
check glucose in 15 minutes; repeat D50W, as above, if necessary
2.
• Gl
ucose 40-59 mg/dL: Give ½ ampule D50W by slow IV push over 30 seconds
• Re
check glucose in 15 minutes; repeat D50W, as above, if necessary
Maintenance
I
V
fl
uids
When blood glucose is:
DK
A: 200 mg/d
L, ch
ange
I
V
t
o
D5 ½ N
S
a
nd run at
mL/hou
r
HHS: 250 mg/dL, change IV to D5 ½ NS and run at mL/hour
Other
:
Fo
r patients at risk of volume overload, consider
D
10
W or D
50
W (Infuse D
50
via central line using infusion pump)
Not
e: HH
S: Ma
intain blood glucose at 250-300 mg/d
L u
ntil plasma osmolarity is ≤315 m
Osm
/Kg
Potassium replacement
Call physician if K is <3 or >6 mEq/L (Note: Urine output should be >30 mL/hour before starting K
+
replacement)
Add K
Cl to I
V
f
luids:
• If K is <3.3 mEq/L, add 30 mEq KCl/L of IV fluid
• If K is 3.3- 5.2 mEq/L add 20 mEq KCl/L IV fluid to maintain K between 4-5 mEq/L
• If K
+
is >5.2 mEq/L, hold KCl
• Consider KPO
4
instead of KCl if serum PO
4
is low
Other:
2
of 3– Orders for Adults with DKA and Hyperglycemic Hyperosmolar State (HHS) – Approved 7/31/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Phosphorus
replacement
Consider if evidence of alcohol abuse, malnutrition, etc.
Give 10 m
Eq/L KPO
4
in one liter of IV fluid x 1
Other
:
S
od
ium bicarbonate (
DKA
)
Give sodium bicarbonate
If pH <
6.9 dilute 100 mmol
NaHCO
3
in 400 mL H
2
O containing 20 mEq KCl
Inf
use over 2 hours
Other
I
V
Pus
h
am
pule of
NaHCO
3
Recheck arterial pH (ABG) within minutes and call results to the attending
Alert parameters
for notifying physician
• Two consecutively treatments for hypoglycemia
• K less than
mEq
/
L
• Wi
thholding IV insulin infusion for >1 hour with no other source of insulin
• TPN stopped, interrupted or any change in formulation
• Deterioration in mental status
• Patient does not respond to above orders for glycemic control
Other
Other
T
ra
nsition to
SQ in
sulin
Pro
ceed to
Tex
as
Dia
betes
Cou
ncil
Tra
nsition Algorithm From
I.V. to S.Q
.
Ins
ulin
Other
:
Other
orders
1.
2.
3.
4.
R
ef
erences:
1.
Am
erican
Dia
betes Association.
Sta
ndards of medical care in diabetes-2008.
Dia
betes
Car
e. 2008;31(
Sup
pl 1):
S12
-
S54
.
2. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in adult patients with diabetes. A consensus statement from the American Diabetes
Association.
Di
abetes
Care
. 2006;29(12):2739-2748.
3.
Am
erican
Dia
betes Association. Hyperglycemic crises in patients with diabetes mellitus (
Pos
ition
Sta
tement).
Dia
betes
Car
e. 2004;27 (
Sup
pl 1):
S94
-
S10
2.
4.
C
le
ment
S, Bra
ithwaite
S, Ma
gee M, et al. Management of diabetes and hyperglycemia in hospitals (technical review).
Dia
betes
Car
e. 2004;27:533-591.
5.
L
ee P, Gr
eenfield J
R, Cam
pbell
L
V
. “M
ind the gap” when managing ketoacidosis in type 1 diabetes.
Dia
betes
Car
e. 2008;31(7):e58.
Physician Signature Date Time
3
of 3– Orders for Adults with DKA and Hyperglycemic Hyperosmolar State (HHS) – Approved 7/31/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Transition Algorithm from I.V. to S.Q. Insulin for
Patients with Diabetes or Hyperglycemia
GOALS: NPO or PO
F
PG 10
0-130 mg/d
L
2h p
p <180 mg/d
L
AC <
140 mg/d
L
Avo
id hypoglycemia
GOALS: TPN or Enteral
<180 mg/d
L
Avo
id hypoglycemia
Transition From I.V. to S.Q. Insulin
1-4
1. Patient’s Total Daily Dose (TDD) = Sum of the previous 4 hours x 5
(
Thi
s will provide ~80% of the current insulin infusion)
Note: If patient was nondiabetic and using <1 unit per hour, insulin can be discontinued
2. Give one-time injection of
Bas
al
Ins
ulin
5-7
+ Bridging Dose
8
of aspart, lispro or glulisine
Bas
al dose =
T
DD
Bri
dge dose = 10% of
T
DD
3. S
top IV insulin infusion
4. Start patient on pathway 1, 2, 3 or 4
1-3
depending on route or number of meals per day
1 Patient will not start eating
Prandial
8
Insulin = None
Basal
5
Insulin = TDD q AM
3 Patient will eat 3x per/day
Pra
ndial
Ins
ulin = ½
TD
D
÷ t.
i.d. A
C
Bas
al
Ins
ulin = ½
TD
D
q A
M
4 TPN or Enteral Nutrition
TP
N
: Use R i
nsulin;
Dos
e = 80%
T
DD
Ma
y add part or all to
T
PN
b
ag
Tube
feeding:
Con
tinuous rate
Bas
al insulin =
TD
D
Inte
rmittent feedings
Bas
al
5
insulin =½ TDD
Prandial
8
insulin =½ TDD÷ t.i.d. AC
Changing Prandial or Basal Insulin
Any glucose <80 insulin 20%
All glucose 80-179
No Cha
nge
Any glucose ≥180 insulin 10%
Correcting Hyperglycemia
• Use prandial insulin q4-6 h
• Dose: see “Changing Prandial Insulin”
Reevaluate Total Daily Dose of Insulin
1. Recalculate the TDD every 1-2 days as
the doses of insulin are adjusted.
2. The ratio of basal to prandial insulin
should be approximately 50:50
Changing Basal
5
Insulin
Adjust Each Morning
F
PG Ins
ulin
Change
<
60 mg/d
L - 4 un
its
60-80 - 2
81-99 - 1
100-130
No Change
1
31-140 + 2
141-160 + 4
161-180 + 6
>18 0 + 8
Changing Prandial
8
Insulin
• Add/subtract to prandial dose if glucose is / before meal
• Use alone to correct any random high glucose
F
PG TDD
<40
units/d
TDD
~ 4
0 - 80 uni ts /d
TDD
>8
0 units/d
<60 – 2 unit – 3 unit – 4 unit
60-99 – 1 – 2 – 2
100-139
No Change
1
40-199 + 1 + 1 + 2
200-249 + 2 + 3 + 4
250-299 + 3 + 5 + 7
300-349 + 4 + 7 +10
>349 + 5 + 8 +12
2 Patient eats <3 meals/day
Eac
h prandial dose =
10
%
T
DD
Bas
al
Ins
ulin: 90%
TD
D
i
f 1 meal
Bas
al
Ins
ulin: 80%
TD
D
i
f 2 meals
Footnotes:
1
www.diabetes.org/for-health-professionals-and-scientists/insulin-administration.jsp,
2
Donaldson S, et al. Diabetes Educator. 2006;32:954
3
Hirsch IB. Insulin. 2006;1(Suppl A):S18-24
4
DeSantis AL, et al. Endocrine Practice. 2006;12:491-505
5
Basal insulin = glargine or detemir
6
If patient is transferred out of the unit in the later evening and will begin eating in the A.M. give
half the basal dose and all of the bridging dose.
Beg
in full basal dose the next morning.
7
If NPH is used, then give 2/3 of the TDD and distribute as 2/3 in the morning and 1/3 at bedtime.
8
Aspart, lispro or glulisine is recommended because the action profiles better approximate normal
physiology.
Reg
ular insulin may be substituted.
1 of 1 – Transition Algorithm from I.V. to S.Q. Insulin for Patients with Diabetes or Hyperglycemia – Approved 7/31/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 7/31/08

1. Tot al Daily Dose (TDD) of S.Q. insulin equals I.V. units insulin used over the last 4 hours x 5
TDD = ( units used over the last 4 hours) X (5) = units insulin
NOTE: If patient was using less than 1 unit insulin per hour, D/C basal insulin
&
use only supplemental insulin if T2 DM
2. Start S.Q. basal insulin 2 hours prior to discontinuing insulin drip
1st basal dose insulin = TDD = units basal insulin
3. Daily insulin regimen (Start Basal-Bolus insulin regimen depending on route or number of meals per day)
TDD
Prandial Insulin Dose
Do not give prandial insulin dose if patient missing meal
Supplemental Dose (CBG = capillary blood glucose)
(see #5, below)
NPO
100% TDD = units basal insulin every 24 hours None Every 6 hours for CBG >140 mg/dL
1 meal per day
80% TDD = units basal insulin every 24 hours 10% TDD = units insulin before meal Before meal and every 6 hours for CBG >140 mg/dL
2 meals per day
70% TDD = units basal insulin every 24 hours 15% TDD = units insulin before each meal Before meals, and bedtime for CBG >140 mg/dL
3 meals per day
50% TDD = units basal insulin every 24 hours 50% TDD ÷ 3 = units before each meal Before meals, and bedtime for CBG >140 mg/dL
4. Monitor capillary blood glucose before meals and bedtime 2 a.m. every 4 hours every 6 hours
5. Correction dose for preprandial or random hyperglycemia
Glucose
mg/dL
High Insulin Sensitivity
<40 units/day
Average Insulin Sensitivity
40-80 units/day
Low Insulin Sensitivity
>80 units/day
Units Insulin to Administer
141-200 1 1 2
201-250 2 3 4
251-300 3 5 7
301-350 4 7 10
>350
5 & call
8 & call
12 & call
6. Titrate basal insulin each morning based on fasting glucose: Increase 2 units if glucose >140 mg/dL
Decrease 2 units if glucose <80 mg/dL
7. Titrate prandial insulin. Use same schedule as in #5, above
8. Recalculate new TDD every 1-2 days based on changes in basal and prandial insulin requirements
9. Remember, the ratio of basal to prandial insulin should be approximately 1:1
1 of 1 – Transition from I.V. to S.Q. Insulin Order Set Eating Status NPO or PO – Approved 10/27/11 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Transition from I.V. to S.Q. Insulin Order Set
Eating Status NPO or PO
GOALS:
Fasting
1
00-140 mg/dL
2 hr postprandial
1
40-180
Before Meals
<
140-180
INSULIN:
IV insulin regular
Basal insulin
g
largine, detemir (or NPH BID)
Prandial
a
spart, glulisine, lispro, regular
Supplemental
a
spart, glulisine, lispro, regular
Diabetes treatment algorithms
Approved 10/27/11

1 of 1 – Transition from I.V. to S.Q. Insulin Order Set TPN or Enteral (Tube) Nutrition – Approved 10/27/11 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
1. Total Daily Dose (TDD) of S.Q. insulin equals units insulin used over the last 4 hours x 5
TDD = ( units used over the last 4 hours) X (5) = units insulin
NOTE: If patient was using less than 1 unit insulin per hour, D/C basal insulin
&
use only supplemental insulin if T2DM
2. Start S.Q. basal insulin 2 hours prior to discontinuing insulin drip
1st basal dose insulin = TDD = units basal insulin
3. Daily insulin regimen
TDD
Prandial Insulin Dose
Do not give prandial insulin dose if patient missing meal
Supplemental Dose (CBG = capillary blood glucose)
(see #5, below)
TPN
100% TDD = units basal insulin every 24 hours None Every 4 hours as needed for CBG >140 mg/dL
Tube (continuous)
100% TDD = units basal insulin every 24 hours None Every 4 hours as needed for CBG >140 mg/dL
Tube (bolus)
50% TDD = units basal insulin every 24 hours 50% TDD ÷ # bolus feeds = units insulin before each bolus Before each bolus as needed for CBG >140 mg/dL
4. Monitor capillary blood glucose before meals and bedtime 2 a.m. every 4 hours every 6 hours
5. Correction dose for preprandial or random hyperglycemia
Glucose
mg/dL
High Insulin Sensitivity
<40 units/day
Average Insulin Sensitivity
40-80 units/day
Low Insulin Sensitivity
>80 units/day
Units Insulin to Administer
141-200 1 1 2
201-250 2 3 4
251-300 3 5 7
301-350 4 7 10
>350
5 & call
8 & call
12 & call
6. Titrate basal and prandial insulin:
Any glucose <80 mg/dL g Decrease insulin 20%
All glucose 80-180 mg/dL g No Change
Any glucose >180 mg/dL g Increase insulin 10%
Transition from I.V. to S.Q. Insulin Order Set
TPN or Enteral (Tube) Nutrition
GOAL:
80-180 mg/dL
INSULIN:
IV insulin regular
Basal insulin
g
largine, detemir (or NPH BID)
Prandial
a
spart, glulisine, lispro, regular
Supplemental
a
spart, glulisine, lispro, regular
Diabetes treatment algorithms
Approved 10/27/11

1 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Introduction
The goal of insulin delivery is to regulate blood glucose levels to achieve normoglycemia. In someone
without diabetes, pancreatic B-cells continuously secrete insulin throughout the day and night,
providing a continuous insulin infusion or basal amount. In response to meals, the pancreas provides
“bursts” of insulin referred to as boluses.
Pump therapy is intended to more closely mimic this pancreatic function. Continuous subcutaneous
insulin infusion (CSII) utilizes only fast acting insulins (Humalog, Novolog) and eliminates the use
of long-acting insulins (NPH, Ultralente, Lantus). Pumps can deliver insulin in 0.1 unit increments
as a basal/continuous flow between meals and through the night. Basal rates can be increased
or decreased at any point, allowing for exercise, illness, skipped meals, sensitivity to insulin and
the dawn phenomenon. Boluses of insulin can be delivered via the pump to provide insulin to
compensate for carbohydrate intake and hyperglycemic episodes when needed.
Insulin pump therapy gives people with diabetes the freedom to enjoy life, despite their chronic
condition. The value of an improved lifestyle, increased flexibility and optimal diabetes control is
obvious from the impact the insulin pump has made in the twenty-five years since its inception.
The ability to control how and when insulin is delivered provides the “pumper” with increased
flexibility in scheduling their day-to-day activities. For those people with erratic lifestyles, a desire
to achieve optimal glycemic control (A1c ≤ 6.5%) and prevent chronic complications, the pump is
an ideal choice.
INDICATIONS FOR PUMP THERAPY
Clinical Indications
1. Inadequate glycemic control with MDI (Multiple Daily Injections) therapy
2. Recurrent severe hypoglycemia
3. Recurrent hyperglycemia
4. Hypoglycemia unawareness
5. Dawn
phenomenon
6. Pr
econception
7. Pr
egnancy
8. Gastroparesis
9. Early neuropathy or nephropathy, when improvement in glucose control can reduce
acceleration of complications
10. Renal
transplantation
Insulin Pump
Therapy
sUPPlement

2 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
11. Frequent DKA
12. Uncontr
olled diabetes
13. Erratic
Blood Glucose
14. Pr
event or delay complications
15. Desir
e to improve lifestyle flexibility
16. A1c greater than 6.5%
Lifestyle indications
1. Erratic schedule
2.
Varied
work shifts
3.
Desir
e for improved flexibility
4.
Inconv
enience of multiple daily injections
Advantages of Pump Therapy
1. More flexible lifestyle
2. Improved overall control
3. Prevent chronic complications
4. Improved control during exercise and “growth spurts”
5.
Tight control during pregnancy
Characteristics of Pump Candidates
Ready, willing, and able
1. Is motivated — pump therapy requires a strong desire to improve one’s health and is a time
investment for weeks or months in advance and during the initiation of pump therapy.
2. Has realistic expectations — a potential pump candidate must understand that the pump will
not “fix” blood glucose variations automatically, nor will it grant freedom from frequent SMBG
(self monitoring of blood glucose).
3.
Demonstrates
independent diabetes management — a thorough knowledge of diabetes and
its management and the ability to demonstrate appropriate self-care behaviors provide the
foundation for advanced self-management skills required by pump users.
4.
Is
practicing counting carbohydrates — has a willingness to practice the Carbohydrate (CHO
or carb) Counting method, and an understanding of insulin actions and pre-meal bolus dosing
calculations.

3 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
5. Has manual dexterity — able to use buttons on the pump and has good visual acuity to see
the
screen.
6.
Has
a good support system — emotional support is crucial to the success of pump therapy.
7.
Demonstrates
emotional stability — a potential pumper must attend education sessions and
attend to tasks that require routine attention. The patient must keep physician appointments.
Poor Candidates for Pump Therapy
1. Patients who are unwilling to comply with follow-up appointments.
2.
Patients
who are unwilling to receive diabetes education.
3. Patients who are unwilling to perform SMBG 8 times a day initially and then, 4-6 times a day
after CSII therapy is established.
4. Patients who are unable or unwilling to count carbohydrates.
DETERMINING TOTAL DAILY DOSE AND BASAL RATE
Method #1:
Pre-pump Total Daily Dose (TDD)
Reduce pre-pump Total Daily Dose by 25%
Divide “pump” TDD in half: 50% for basal; 50% for bolus
Method #2:
Using Patients Weight Factor: Weight (lbs) X 0.1 = basal rate per hour
Start with 1 basal rate per 24 hours.
Based on blood glucose results during the times listed below, it may be necessary to implement
additional basal rates based on patient’s blood glucose (BG)
12:00 midnight – 3:00 a.m.
3:00 a.m. – 7:00 a.m.
7:00 a.m. – 12:00 noon
12:00 noon – 6:00 p.m.
6:00 p.m. – 12:00 midnight
Time Frame For Beginning Pump Therapy
1. 1–2 months before pump start:
u
Assess whether or not patient meets the criteria for a “pumper.”

4 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
u
MD writes orders for insulin pump therapy. Contacts the insurance company for
pre
-au
thorization of coverage.
u
Patient is seen by a CDE/dietitian for carbohydrate counting instruction.
u
Patient is seen by the pump trainer for knowledge assessment and education as needed —
to include: hypoglycemia, hyperglycemia and sick day management, prevention of DKA,
patient’s responsibilities, and general knowledge regarding diabetes.
2. 1-2 weeks before pump start:
u
Patient watches video/DVD on use of the pump several times to familiarize him/herself with
the pump.
u
May attend “pump school” via the Internet if available.
u
Meets with pump trainer for basal rates, boluses, insulin to carbohydrate ratio, and insulin
correction factor if not already done.
3. Day before pump start:
u
Discontinue use of long-acting insulin (NPH, Lantus, Ultralente).
u
Continue injecting Novolog or Humalog before meals.
u
Use “correction formula” to cover for “highs.”
4. Day of pump start:
u
Eat breakfast and inject Humalog or Novolog as usual.
u
Wear comfortable, loose-fitting clothing — preferably 2-piece outfit.
u
Allow 3 hours for training.
u
Bring supplies with you to include:
Pump, User’s Manual, Infusion Sets — at least 2, Cartridges — at least 2, Skin Prep,
Glucose Meter / Lancets / Strips, Alcohol Wipes, Insulin (Novolog or Humalog),
Batteries, Carbohydrate Snack.
5. First Day of Pump Therapy:
u
Begin “Four-Day Plan.”
u
Call pump trainer with glucose levels and carbohydrate intake.
6. When “Four-Day Plan” completed:
u
Come into office for first follow-up. Patients MUST bring: documentation of glucose
readings, boluses (for elevated glucoses or meals), diary of carbohydrate Intake.
u
Begin “Three-Day Plan.”
7. Within 1–2 days after completing “Three-Day Plan”
u
Call pump trainer with readings.
u
Adjust basals/boluses as needed.

5 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
8. Weekly for four weeks:
u
Call pump trainer and report complete record.
u
Adjust basals, insulin to carbohydrate ratios as needed.
u
Instruct on added features of the pump, i.e., Dual and Square Wave Boluses, utilizing
temporary basal rate, Easy Bolus, Audio Bolus.
u
Adjust basal rates first, based on fasting glucoses. When fasting glucoses are at goal, adjust
boluses and/or insulin to carbohydrate ratios to achieve pre- and post-meal glucose goals.
TESTING BASAL RATES: FOUR DAY PLAN
First Day
1. Eat supper by 7 p.m.
2.
Skip a bedtime snack.
3
.
Te
st blood sugar every 2 hours between supper and bedtime; at 12:00 Midnight,
and at 3:00 a.m.
4.
Re
cord your results!
Second Day
1. Eat breakfast.
2. Skip lunch.
3. Test blood sugar every 2 hours between breakfast and supper.
4. Record your results!
Third Day
1. Skip breakfast.
2. Test blood sugar every 2 hours between waking up until lunch.
3. DO NOT SLEEP IN!
4. Record your results!
Fourth Day
1. Skip supper.
2. Test blood sugar every 2 hours between lunch and your bedtime snack at 10:00 p.m.
3. Record your results!
NOTE: Do not “fix” a high blood sugar during the time you are checking every 2 hours. Correct at
your next scheduled meal using your correction factor.
If you miss a day, continue the plan the next day. But try not to miss a day — the sooner the plan is
completed, the sooner your basal rates will be set.

6 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
PRE-PUMP EDUCATION CHECKLIST
Patient Name
____________________________________________
Date
___________________
Certified Pump Trainer
___________________________________________________________
MD’s Name
____________________________________________________________________
Pump Model
____________________________________
Serial #
________________________
UNDERSTANDING PUMP THERAPY NUTRITION
£ Theory
£ Insulin Type
£ Basal Rate
£ Meal Bolus
£ Insulin Sensitivity/
Correction Factor
£ Carb. Counting
£ Using Food Labels
£ Insulin to Carb. Ratio
£ Proper Snacks
BLOOD GLUCOSE TESTING EXERCISE
£ Schedule
£ Alc
£ Safety
£ Hypoglycemia
£ Proper Snacks
£ BG Checks
HYPOGLYCEMIA PUMP THERAPY RESOURCES
£ Protocol/”Rule of Fifteen
£
G
lucagon
£ User’s Guide
£
Pu
mp School Online
£
We
bsites
HYPERGLYCEMIA WHEN TO CALL YOUR DOCTOR
£ Protocol
£ Ketone Testing
DKA WHEN TO CALL 24 HOUR HELP LINE
£ Causes
£
Sig
ns and Symptoms
£
P
revention
SICK DAY MANAGEMENT
£ Protocol
£ Supplies
Notes:

7 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
DETERMINING BOLUSES
Calculating Insulin Sensitivity Factor (ISF)
Also may be referred to as the Insulin Correction Factor (ICF)
The Insulin Sensitivity Factor (ISF) is the amount of blood glucose reduced by 1 unit of rapid or short
acting insulin over a 2–4 hour period. Two commonly accepted formulas are used to determine the
ISF: the 1800 Rule and the 1500 Rule. Endocrinologist Paul C. Davidson, MD developed the 1500
Rule. With the introduction of rapid-acting insulin, John Walsh, PA CDE modified the 1500 Rule
into the 1800 Rule. Generally, the 1800 Rule is used for patients who are insulin sensitive or those
who use rapid-acting insulin and the 1500 Rule for patients who are insulin resistant or those who
use short-acting insulin. The Rules calculate the ISF by dividing either 1800 or 1500 by the TDD.
Amount of Blood Glucose lowered by 1 unit of insulin (1800 Rule)
1800 = ISF
TDD
Note: 1800 currently used with Humalog or Novolog instead of 1500 (1500 Rule)
Calculating Insulin to Carb Ratio (ICR)
This method of determining the Insulin:Carbohydrate ratio is based on Total Daily Insulin Dose
(TDD). The TDD is divided into 500 and the result is the amount of carbohydrate that one unit
of rapid- or short-acting insulin will cover. The goal is to bring blood glucose levels into the target
range 3–4 hours after the meal.
Grams of carbs covered by 1 unit of insulin (500 Rule)
500 = ICR
TDD
TYPES OF BOLUSES
Normal Bolus—total bolus infused at onset of meal
Square Wave—total bolus infused slowly over several hours; useful in cases of gastroparesis
Dual Wave—part of bolus is infused at onset of meal, and remainder is infused slowly over several
hours; useful for high fat meal, i.e., pizza, Mexican food.
Adjusting/Fine Tuning Dosage
Empower patients to evaluate and adjust their BG. Resume intensive monitoring if necessary, i.e.,
8 times a day. Start with overnight basals; promote low-fat, consistent carb content meals. Introduce
high fat meals after ICR has been established or corrected. When high fat meals are consumed,
consider utilizing Dual Wave bolus. Two-hour postprandial glucose goals should be 30 +/- points
above preprandial BG. Patient may require a different ICR for each meal. BG targets should be
determined by the provider and the patient and depending on age of the patient, concomitant

8 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
conditions and the patients’ ability and willingness to achieve tight control of their diabetes.
POSSIBLE COMPLICATIONS OF PUMP THERAPY
Hypoglycemia — fewer episodes than with MDI. Possible improvement in hypoglycemic
unawareness.
Diabetic Ketoacidosis — interruption in Humalog/Novolog delivery can lead to high BG and DKA
in 4 +/- hours. Patient must check BG 4–6 times a day.
Skin Infections — meticulous skin care is necessary at infusion sites, which must be rotated every
2–3 days.
Weight Gain — could be a result of improved control or if patient liberalizes diet.
Initiation of CSII should be done by a Certified Pump Trainer (CPT) who is usually provided by the
insulin pump manufacturer, or a Certified Diabetes Educator (CDE), who has received specialized
training in insulin pump therapy. The various features of the pump should be demonstrated/
explained to the patient who should be provided with phone numbers of the insulin pump company
and the provider. The patient should be encouraged to keep detailed records of BG, insulin dosage,
carb intake, and other daily activities.
Table for Estimated Basal Rate and Insulin to Carbohydrate Ratio
WEIGHT IN POUNDS BASAL INSULIN CARBOHYDRATE RATIO
100 0.3 to 0.5 1 unit / 16 gms
110 0.3 to 0.5 1 unit / 15 gms
120 0.4 to 0.6 1 unit / 15 gms
130 0.4 to 0.6 1 unit / 14 gms
140 0.5 to 0.7 1 unit / 13 gms
150 0.5 to 0.7 1 unit / 12 gms
160 0.6 to 0.8 1 unit / 12 gms
170 0.6 to 0.8 1 unit / 11 gms
180 0.7 to 0.9 1 unit / 10 gms
190 0.8 to 1.0 1 unit / 9 gms
200 0.9 to 1.1 1 unit / 8 gms

9 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Estimated Correction Factor
CURRENT TDD CORRECTION FACTOR
10 units 150 points
20 units 75 points
25 units 60 points
30 units 50 points
40 units 38 points
50 units 30 points
60 units 25 points
75 units 20 points
100 units 15 points
150 units 10 points
Carbohydrate Counting
Carbohydrate counting is a meal planning approach that works well with insulin pump therapy. It
is a great way to add variety and flexibility in choices of meals and snacks. Carbohydrate counting
has been proven to help achieve better glucose control.
Generally carbohydrate is the main food group that increases blood sugar. Protein has a sustaining
effect and fat slows absorption.
It is essential that the patient understands and practices the techniques of carbohydrate counting
prior to pump initiation.
Many references such as the materials included in Chapter 5 of Diabetes Life Skills Book or the
“Daily Meal Planning Guide” by Eli Lilly are used by the CDE or Registered Dietitian to teach
Carbohydrate Counting.
Tools needed to count carbs:
1. Measuring cups
2. Food labels
3. Calculator
4. Carb counting book/guide
Carbohydrate containing foods include breads, pasta, rice, other grains, starchy vegetables (potatoes,
corn, peas), crackers, cereals, fruit (fresh, canned, frozen, or juice), milk, yogurt & ice cream, cooked
dried beans, cake, cookies, pie, sugar/honey.
One serving is considered 15 grams of carbohydrate and is contained in:
1/3 cup cooked rice, beans, or pasta
1/2 cup starchy vegetables like corn, peas, potato, or cooked cereal

10 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
1 slice bread or 1 tortilla
1 small
piece of fruit, ½ small banana, or ½ cup light canned fruit
1 cup
milk
Using
measuring cups and reading labels are highly recommended as the patient practices
at home.
THE RULE OF 500
This method of determining the Insulin:Carbohydrate ratio is based on Total Daily Insulin Dose
(TDD). The TDD is divided into 500 and the result is the amount of carbohydrate that one unit
of rapid- or short-acting insulin will cover. The goal is to bring blood glucose levels into the target
range 3–4 hours after the meal.
Example:
TDD is 36 units
Glucose levels are within target range
500/36 = 13.8 (round up to 14 or 15)
Insulin to carbohydrate ratio is 1:15
1 unit of insulin covers 15 gm carbohydrate
Some CDEs find that dividing 450 (rather than 500) by the TDD is more accurate for short-
acting insulin and/or for people who are more insulin resistant.
Insulin Sensitivity
The Insulin Sensitivity Factor (ISF) is the amount of blood glucose reduced by 1 unit of rapid or short
acting insulin over a 2–4 hour period. Two commonly accepted formulas are used to determine the
ISF: the 1800 Rule and the 1500 Rule. Endocrinologist Paul C. Davidson, MD developed the 1500
Rule. With the introduction of rapid-acting insulin, John Walsh, PA CDE modified the 1500 Rule
into the 1800 Rule. Generally, the 1800 Rule is used for patients who are insulin sensitive or those
who use rapid-acting insulin and the 1500 Rule for patients who are insulin resistant or those who
use short-acting insulin. The Rules calculate the ISF by dividing either 1800 or 1500 by the TDD.
Example:
TDD is 34 units
1800/34 = 52.9
ISF is 52.9. One unit of rapid-acting insulin decreases glucose by 52.9 mg/dL
This can be rounded to 55
Another method of calculating the ISF is to use the general “safe” starting point of 1 unit:
50 mg/dL. This method may work well with most lean to average adults.

11 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
An alternative method for Insulin:Carb ratio can be figured once the person’s ISF is calculated,
multiplying it by 0.33 provides an insulin-to-carbohydrate ratio.
Example:
ISF is 55 mg/dL
55 x 0.33 = 18.15 (round to 18)
Insulin to carb ratio is 1:18
1 unit of insulin covers 18 g of carbohydrate
Verifying Insulin:Carb Ratio and Insulin Sensitivity
Prior to eating, the bolus insulin dose is partially based on the insulin to carbohydrate ratio. This
ratio tells how many grams of carbohydrate are affected by one unit of insulin. The ratios can be
verified with one of the methods described below:
Method 1: Food diary, insulin dose, and SMBG information
The pump user is to keep 3 days of records, including:
1.
Fa
sting, pre-meal, and 2-hour PPG results
2.
Pr
e-meal insulin doses
3.
Am
ount of carbohydrate consumed at meals and other times. It is helpful if the patient
consumes the same amount of carbohydrate at each breakfast for 3 days, same amount of
carbohydrate at each lunch for 3 days, etc.
4.
Am
ount of all food and beverage consumed, as fat and protein moderately affect blood sugar.
With these records, determine the amount of insulin the patient used to cover the carbohydrate
consumed at each meal by dividing the total grams of carbohydrate by the number of units of insulin.
Example:
Co
nsumed 60 g carbohydrate
Injected (bolused) 5 u rapid-acting insulin
PPG is within 30 mg increase of pre-meal blood glucose
60/5 = 12
Insulin to carbohydrate ratio = 1:12
1 unit of insulin covers 12 g carbohydrate

12 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
CARBOHYDRATE COUNTING FOOD LOG
Write down all food or drink you consume for at least 3 days. Be sure to include portion sizes and
the time you eat or drink. Estimate the amount of carbohydrates in each meal and snack; then
record the amount of insulin you took. Bring this log with you on appointments to the pump trainer
or the dietitian.
DATE/TIME
BLOOD SUGAR
(2 HRS PP) FOOD GRAMS OF CARBS INSULIN
DATE/TIME
BLOOD SUGAR
(2 HRS PP) FOOD GRAMS OF CARBS INSULIN
DATE/TIME
BLOOD SUGAR
(2 HRS PP) FOOD GRAMS OF CARBS INSULIN

13 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Calculating Total Grams of Carbohydrate in a Recipe
To determine the amount of carbohydrates in a recipe:
1.
Make
a table as noted below
2.
List ALL
the ingredients in the recipe
3.
Using
food labels or a nutrient composition book, list the total grams of carbohydrate in
each ingredient (amount of fat and sodium can also be calculated)
4.
Total
the grams of carbohydrate from all ingredients
5. Divide the total grams of carbohydrate by the number of servings in the recipe
6.
Note
the total grams of carbohydrate PER SERVING on the recipe for future reference
Recipe Name:
__________________________________________________________________
Ingredient Amount
Grams of
Carbohydrate
Grams of Fat
Example:
Corn Pudding (Makes 8 Servings)
Ingredient Amount
Grams of
Carbohydrate
Grams of Fat
Cornstarch 2 Tablespoons 14 0
Egg Substitute ½ cup 2 0
Sugar ½ cup 100 0
Creamed Corn 16 oz. can 60 0
Evaporated Skim Milk 16 oz. can 60 0
TOTAL 236 0
Divide total carbohydrate by number of servings (236/8) 45
This recipe has 29.5 grams of carbohydrate and zero (0) grams of fat per serving.

14 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
IDENTIFYING AND MANAGING HYPERGLYCEMIA
Sick Day Management (Refer to “Sick Day Guidelines” in TDC Tool Kit)
During periods of illness, it may be more difficult to maintain good control of blood glucose.
Examples of illness or “sick days” include: dental surgery, colds, sore throat, mild infections, nausea,
vomiting, diarrhea, or fever. It is important to monitor blood glucose more frequently during a sick
day and to take immediate action to prevent ketoacidosis.
Guidelines to follow:
Medication
Never omit insulin. Even if unable to eat, insulin need continues and may increase.
Continue the basal dose of insulin and make additional corrections using the Correction/Sensitivity
Factor as needed. Urine ketone testing can further guide the correction doses.
Blood/Urine Testing
Check blood glucose before usual mealtimes and every 2 to 4 hours, keeping a written record
of results.
Check urine for ketones if blood glucose is greater than 250 mg/dL or as directed by the physician.
Fluids/Meal Planning
Consuming adequate fluids is important during illness. Drink fluids every hour while awake and
during blood glucose checks at night.
If able to eat, drink non-caloric beverages.
If unable to eat, alternate non-caloric beverages with those containing carbohydrate.
Consume 10–15 grams of carbohydrate every 1–2 hours.
Severe high blood glucose and ketoacidosis (DKA) are serious medical problems that sometimes
occur in diabetes. High blood glucose can exist for some time without triggering ketoacidosis.
Ketoacidosis begins only after insulin levels in the body go very low. When insulin is low, glucose
cannot be used as fuel. Glucose is the body’s first choice for energy, but if not available due to
inadequate insulin levels, the body must start burning fat even though glucose is high in the blood.
Ketones are the by-product of burning fat for energy and in high levels, cause nausea and vomiting.
Vomiting, in combination with high blood sugars, can lead to dehydration.
Ketoacidosis can be triggered by:
1. Illness
2. Infections
3. Pump Malfunction
· Loose Luer-lock connection

15 o f 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
· Dislodged infusion set
· Site irritation or overuse
· Empty pump reservoir/cartridge
· Expired insulin
· Incorrect bolus calculation
· Missed bolus doses
· Inadequately programmed basal rates
A pump user needs to take a correction dosage using a syringe if spilling moderate to large ketones,
then change the infusion set. Plenty of water should be consumed to help flush ketones from the body.
Call a physician for further instruction.
IDENTIFYING AND MANAGING HYPOGLYCEMIA
Causes:
Glucose levels can drop to dangerously low levels if there is not a balance between food, medication,
and activity. It can occur very quickly and without warning. Not eating properly, delaying or
skipping meals, an error in medication dose, or engaging in exercise that is too difficult or too
strenuous are all causes of hypoglycemia.
Signs and Symptoms:
Shaking Sweating
Weakness Anxiety
Headache
Bl
urred vision
Dizziness
Fa
st heartbeat
Irritability
Fa
tigue
“Rule of 15”
1. Immediately stop activity and check glucose levels. If driving, immediately pull off the road
2. If no glucose meter is available, treat regardless
3. Consume 15 gms of a fast-acting carbohydrate
•½cupjuice •5sugarcubes •4glucosetablets
•6–7lifesavers •½cupregularsoda •8oz.skimmilk
•2tsp.sugar •8–9jellybeans •1tubeglucosegel

16 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
4. Rest for 15–20 minutes
5. Retest
glucose — if still below 70 mg/dl, repeat fast-acting carbohydrate. Or if no glucose
meter is available, and symptoms are still present, repeat fast-acting carbohydrate
6. Continue steps
1 – 5 until glucose level is above 70 mg/dl
7. An extra snack consisting of a carbohydrate and protein may be needed if more than one
treatment was required and no meal will be eaten within a half-hour. Examples are:
½
sandwich
Ch
eese and crackers
Peanut butter and crackers
8. If
several hypoglycemic episodes occur at the same time over a few days, the basal rate will
need to be adjusted; notify the pump trainer immediately
9. ALW
AYS carry a fast-acting carbohydrate in a place that is easily accessible
10. ALW
AYS wear identification stating that you have diabetes and are being treated with an
insulin pump

17 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
APPENDIX
PHYSI
CIA
N’S
ORD
ERS
FOR INS
ULIN PUM
P STA
RT
Patient Name
____________________________________________
Date
___________________
Certified Pump Trainer
___________________________________________________________
These orders expire on
____________________________________________________________
Basal rates may be adjusted by 0.05 increments for BG above
_________
and/or
_________
below.
Starting Basal Rate:
Profile Time Units per Hour
#1
1
2:00 a.m.
____________
Starting Bolus Doses
Insulin to Carbohydrate Ratio:
1 u
nit per
______________
gms. carbohydrate
Insulin Sensitivity Ratio
(Correction Factor):
1 u
nit of insulin will lower BG by
_______
mg/dl
Target Blood Glucose Levels
3:00 A.M.
_________
to
_________
Fasting.
_________
to
_________
Before meals.
_________
to
_________
After meals.
_________
to
_________
Additional Instructions:
Physician’s Signature
_____________________________________________________________

18 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
PATIENT INSULIN PUMP CONTRACT
Patient Name
____________________________________________
Date
__________________
Physician
______________________________________________________________________
I understand, as the patient, it is my responsibility to:
1. Maintain
open communication with my physician, dietitian, and diabetes educator. This will
include recording and reporting my glucose levels, carbohydrate intake, exercise, boluses, basal
rate changes, and other information requested.
2. Per
form glucose testing as requested.
3. I will
change my infusion set every 2 to 3 days and follow the guidelines as set forth for proper
pump management.
4. If hospitalized, I will bring all the needed equipment from home to ensure I have enough
supplies. If I do not have the supplies, it is my responsibility to make arrangements to
obtain them.
5. I will follow the formulas for meal boluses and correction factors prescribed to me by my
physician and/or diabetes educator.
6. I will respond quickly and correctly to hypoglycemia and will report these to my health care
team. I understand the “Rule of 15” to treat a low glucose with 15 grams of a fast-acting
carbohydrate, retest in 15 minutes, and repeat the sequence if necessary.
7. I will respond quickly to hyperglycemia and prevent DKA by following the rules for sick-day
management using my correction factor. I will report to my diabetes care team as needed,
increase the frequency of monitoring, and test my urine for ketones if my glucose is over 240
mg/dl for 2 consecutive glucose readings.
8. I will not disconnect from the pump for longer than an hour. If I desire a “vacation” from
the pump, I will first discuss this with my diabetes care team before doing so and follow their
recommendations.
9. If I am having any difficulty with either pump use or carbohydrate counting, I will
immediately call my diabetes care team for the proper assistance.
10. I will make sure that I have the proper supplies on hand at all times and that it is my
responsibility to reorder supplies as I need them. I will also carry “emergency supplies”
with me at all times, including syringes, in case my site becomes dislodged. I will also wear
identification stating that I have diabetes and wear an insulin pump. This information will also
include emergency contact, my doctor’s name, and telephone number.
Patient’s Signature
________________________________________
Date
__________________

19 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
LETTER OF MEDICAL NECESSITY
Date
________________________________
RE: Patient Name
_____________________________________
Phone ( )
_______________
Patient’s date of birth
___________________
Insurance identification #
____________________
To whom this may concern:
This letter serves as prescription and letter of medical necessity for the above referenced patient for
an insulin infusion pump as a lifetime need.
Check the following:
£ Patient has had diabetes for
____
years
£
Patient
has the ability to regularly monitor blood glucose
____
to
___
times per day.
£ Patient is motivated to achieve and maintain glycemic control and has the support needed to
stay motivated.
£ Patient demonstrates compliance with dietary regimen.
£ Patient’s insulin regimen consists of
______
to
______
injections per day.
£ Patient has attempted several different regimens and/or has had multiple dose changes.
£ Patient uses the following type(s) of insulin:
_________________________________________
.
Patient exhibits one or more of the following:
£ A1c level
___
% on
___
/
___
/
_____
.
£ History of severe glycemic excursions and/or £ Nocturnal Hypoglycemia
£ Hypoglycemia unawareness £ Extreme insulin sensitivity or low insulin req.
£ Widely fluctuating blood glucose levels before meals. (e.g., pre-prandial BG levels commonly
exceed 140 mg/dl and/or are below 70 mg/dl. The range of these blood glucose levels is from
_____
to
_____
.
£
Dawn
Phenomenon where fasting blood glucose often exceeds
______
mg/dl.
£ Day to day schedule variations such as meal times, work schedules or activity level confound the
degree of regimentation required to self manage glycemia with Multiple daily injections.
£ Patient has been hospitalized or needed emergency assistance due to his/her diabetes.
£ Patient has frequent hypoglycemic episodes, up to
_____
times per week.
£ Pregnancy or preconception with a history of poor glycemic control.
£ Secondary complications requiring tighter glycemic control to slow or stop progression of
£ Retinopathy £ Neuropathy £ Nephropathy £ Other:
_______________________
£ Sub-optimal glycemic and metabolic control post-renal transplant.
£ Patient has been fully informed of the risks and benefits of pump therapy.

20 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
PHYSICIAN NOTES
I certify that this information is complete and correct.
__________________________________
I am an endocrinologist, internist or diabetes specialist: £ Yes £ No
I am prescribing an insulin infusion pump, insulin pump supplies, and diabetes supplies for
the following patient. The supplies may be refilled as necessary for one year. Please dispense
as written.
Physician name
Physician street
Physician city, state, ZiP
Physician signatUre
meDica
l
licen
se
nUm
ber
UP
in nUmber
Patient name
Patient street
Patient cit y, state, ZiP
Date
Physician’s Signature

21 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
INSURANCE COVERAGE FOR INSULIN PUMP THERAPY
Private Insurance
1. Contact pump company with information about the patient
A.
In
surance information
B. Indications that would require utilizing the insulin pump
C.
Mu
st be on multiple insulin injections (2 or more a day)
D. Cover type 1 and some type 2 diabetes
E.
Pr
escription from MD
Medicare
1. Contact pump company with patient’s information
2. Must meet criteria for insulin pump therapy
A. C-Peptide of less than 0.6 mcg/L
B. A1c over 7%
C. Monitoring 4 times a day
3.
Medicar
e pays 80% for pump and supplies. Secondary insurance may cover the other 20%. If
Medicare denies coverage, secondary may cover.
Medicaid
1. Contact pump company with insurance information
2. Must meet criteria for insulin pump therapy
2. Prescription from MD
4.
Medicaid
will cover 100%
Indications for Insulin Pump Therapy
1. Unable to normalize glucose levels
A. Erratic glucose excursions
B. A1c over 7%
2. Severe episodes of hypoglycemia or hypoglycemia unawareness
3. Preconception/pregnancy
4. Early chronic complications
5. Organ transplant
6. Patient desires better control
7. Prevent chronic complications

22 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
OVERVIEW FOR PUMPING INSULIN
Indications For Insulin Pump
1. Multiple episodes of severe hypoglycemia
2.
Er
ratic glucose levels – “brittle diabetes”
3. Early complications
4.
Or
gan transplant
5. Pregnancy
Advantages of The Pump
1. More flexible lifestyle
2.
Imp
roved overall control
3. Prevent chronic complications
4. Improve control during exercise and “growth spurts”
5. Tight control during pregnancy
Characteristics of Pump Candidate
1. Must be willing to monitor BG several times a day
2. Must be willing to count carbohydrates
3. Must have manual dexterity to use buttons on pump and have good visual acuity to
see the screen
4.
Good
support system
5. Committed to self-care
6. Ability to problem solve
7. Good basic knowledge of diabetes
8. Reasonable expectations of what the pump can do
Time Line
1–2 months before pump start:
1. Assess patient’s current knowledge about diabetes
2. Assess whether or not patient meets the criteria for a “pumper”
3. MD contacts the pump company and writes orders for the pump
4. Patient is seen by dietitian for carbohydrate counting
5. Patient is seen by the pump trainer for general assessment and education

23 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
QUESTIONNAIRE
Are you ready for pumping?
1. How motivated are you to achieve good control?
Not very
0 1 2 3 4 5 ve
ry
2.
Ho
w many times do you test every day?
0 1 2 3 4 5
3.
Ho
w many injections per day?
0 1 2 3 4 5
4.
Do y
ou keep a record?
Yes (5 points) No (0 points)
5. Do you adjust your insulin for test results?
Ye
s (5 points)
No
(0 points)
6.
Do y
ou adjust your insulin for meals?
Yes (5 points) No (0 points)
7. Do you adjust insulin for “highs”?
Ye
s (5 points)
No
(0 points)
8.
Do you adjust your insulin for exercise?
Yes (5 points) No (0 points)
9. Do y
ou get regular A1c tests?
Yes (5 points) No (0 points)
10. Do you call your doctor when you have a problem?
Ye
s (5 points)
No
(0 points)

24 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
SCORING
Score What It Means
0–9 Are you in charge or someone else?
10–19
At
least you’re honest!
20–29
Wh
ere can you improve?
30–39 Just a few minor changes
40–49
Ho
w soon can you start?
1–2 weeks before pump start
1. Patient watches video or DVD on pump use several times to begin familiarizing him/
herself with the pump
2.
Ma
y attend “Pump School” via Internet
3.
Me
ets with pump trainer for basal, bolus, correction factor, and insulin to CHO ratio
Day before pump start
1. Discontinue use of long-acting insulin
2. Continue injections of Humalog/Novolog before meals
3. Use “correction formula” to cover for highs
Day of pump start
1. Eat breakfast and take fast-acting insulin as usual
2. Wear comfortable clothing-preferably two-piece outfits
3. Allow 3 hours for training
4. Bring with you:
Pump
User’s Manual
Infusion sets — at least 2
Cartridges — at least 2
Skin prep
Glucose meter/strips/lancets
Alcohol wipes
Insulin (Novolog or Humalog)
Carbohydrate snack
2 Batteries

25 o f 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
First day after beginning pump therapy
1. Call Pump Trainer with glucose readings and grams of carbohydrate
2.
Be
gin “4 Day Plan”
Within 3–5 days after pump training
1. Come in to office for follow-up
2.
Co
ntinue “4 Day Plan” until basal rates are adjusted correctly
When basal rates correct,
1. Adjust insulin to carb ratio
2.
Be
gin “3 Day Plan”
3. Call Pump Trainer with BG readings and CHO grams
Weekly for 4 weeks
1. Call Pump Trainer with BG’s and CHO grams for adjustment
2. Basals are adjusted first, then boluses
STARTING BEGINNING BASAL RATE
Total Daily Pre-pump Insulin x 75% = Total Daily Insulin per Pump
(t
otal pre-pump dose minus 25%)
Divide the new dose by 2
Half is basal; half is boluses
For basal, divide half by 24 = basal rate per hour
Begin with 1 basal rate and adjust as needed
Example:
TD
D pre-pump — 50 units
50
– 25% = 38 — new dose
38 ÷ 2 = 19 (19 units for boluses; 19 units for basal)
19
÷ 24 = 0.79 units per hour (may round up to 0.8 units per hr.)

26 o f 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
INSULIN TO CHO RATIO: RULE OF 500
Divide 500 by the new total daily dose:
E
xample:
TD
D = 25 units
50
0 ÷ 25 = 20 — 1 unit of insulin per 20 gms of CHO
TD
D = 45
500 ÷ 45 = 11 — 1 unit of insulin per 11 gms of CHO (may round down to 10 for ease)
INSULIN CORRECTION FACTOR: RULE OF 1500
Divide new TDD into 1500
E
xample:
TD
D = 45 units
15
00 ÷ 45 = 33 (amount I unit of insulin will decrease glucose level by)
If
target level is 100 and glucose level 289 mg/dL – how many units to get BG level to 100?
28
9 – 100 = 189 (189 points above target)
18
9 ÷ 33 = 5.7 units of insulin
Us
ed to correct for a high
Ma
y be added to regular mealtime bolus if high occurs right before eating a meal
MONITORING SCHEDULE
For first few days to 2 weeks (or until basals and boluses adjusted)
1. Between 2:00–3 a.m. (Dawn Phenomenon)
2. Fasting (overnight basal) Goal 70 – 100 mg/dL
3. 2 hours after each meal Goal 140 mg/dL or less
4. Before and after exercise
5. Before driving
6. If hypoglycemia is suspected
ADJUSTING BASALS – “4 DAY PLAN”
Overnight Basal
1. First basal to be checked
2. Eat regular dinner (no later than 7:00 p.m.), NO bedtime snack

27 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
3. BG @ bedtime should be 100-150 mg/dL
4.
Te
st BG every 2 hours between supper and bedtime, @ Midnight, and 3:00 a.m.
5. If BGs stay within 30 mg/dl basal OK — if more than 30, adjust
6.
Di
vide night into 3 “test windows”
a. BEDTIME: 9:00 P.M. to midnight
b.
NIGHT: Midnight
to 3:00 a.m.
c.
DAWN: 3:00
a.m. to 7:00 a.m.
Afternoon Basal
1. Eat breakfast and take bolus for food
2.
NO
lunch, NO bolus
3. Check BG every 2 hours between breakfast to supper
4. If BGs stay within 30 mg/dl, basal OK; if not, adjust
Morning Basal
1. NO breakfast, NO bolus
2. Test BG every 2 hrs from waking until lunch. DO NOT SLEEP IN!
3.
If
BGs stay within 30 mg/dl, basal OK; if not, adjust
Evening Basal
1. NO supper NO bolus
2. Test BG every 2 hrs between lunch & bedtime snack at 10:00 p.m.
3. If BGs stay within 30 mg/dl, basal OK; if not, adjust
NOTE — DO NOT “fix” a high glucose during the time you are checking your BGs every 2 hours.
Correct at the next scheduled meal, using your correction factor. If you miss a day — continue the
plan the next day. May need to repeat the “4 Day Plan” two or three times until the basal rates are
corrected.
ADJUSTING INSULIN TO CHO RATIO
1. Check 2 hours after each meal
2. If BGs not over 140 mg/dL, ratio correct; if higher — increase, if lower — decrease
3. May have 2-3 different ratios during the day — may need 1 unit per 8 gms in a.m., 1 unit
per 10 or 15 for lunch and dinner, or 1 per 8 in a.m., 1 per 10 for lunch, and 1 per 15 for
dinner.

28 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
ADJUSTING CORRECTION FACTOR
1. If hypoglycemia occurs after correcting for a high, lower correction factor
2.
If
BG still high after 3–4 hours, increase factor.
OTHER TIPS AND SAFETY
1. Change site every 2–3 days (every other day with pregnancy). ALWAYS do site changes in
the MORNING — NEVER at bedtime! Check BG 2 hours after a site change to ensure
the “cath” is placed correctly and pump is functioning properly
2. Inspect site twice a day — if swelling, redness, pain, or drainage — CHANGE SITE!
3.
AL
WAYS carry extra supplies with you in case the catheter gets dislodged
4. ALWAYS have a supply of syringes on hand in case of pump malfunction
5.
AL
WAYS wear identification stating you have Diabetes and wear an insulin pump
6.
If you have 2 BGs over 240 mg/dL in a row — inject insulin according to the correction
factor and CHANGE SITE. Retest 2 hours after
7. NEVER NEVER NEVER go to bed with a low battery
8.
If
you perspire heavily, may use a solid non-fragrance antiperspirant around site or try other
types of tape that are available. Skin Tac “H”, Polyskin, Tegaderm, Hypafix, HyTape,
Dermicell, SkinPrep, Mastasol, and toupee glue are other options to try.
GOING OFF THE PUMP
1. Be sure you check with your doctor before disconnecting from the pump for any length of
time.
2. DO NOT disconnect for more than 1-2 hours unless you have the OK from MD.
3. Reasons to go off the pump may be due to pump malfunction — call 1-800-send pump
— the pump manufacturer will immediately send a loan pump until yours is repaired or
replaced. Another reason may be just a desire to have a “vacation” from the pump.
Time Off Pump Action
1
–1½ hrs
No
action unless CHO will be eaten or BG is high
1½–5 hrs. Before disconnecting, give a bolus to replace 80% of
the basal that will be lost
Inject before eating using insulin to CHO Ratio
DAYTIME ONLY
Gi
ve injection before each meal by using your insulin
to CHO ratio PLUS the basal insulin needed until the
next meal
3–4 Days or More
In
ject fast-acting insulin before each meal using your
insulin to CHO ratio and correction factor for highs.
At bedtime, inject Lantus to equal 1.5 X the basal rate
used for the overnight period.

29 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
TRAVELING
1. ALWAYS carry at least 1 weeks’ worth of extra supplies on top of what you will normally
use — if you are staying for 2 weeks, carry supplies for 3 weeks.
2.
NE
VER check your supplies in baggage — CARRY them with you.
3. Carry snacks with you.
4.
WE
AR IDENTIFICATION stating you have diabetes and wear an insulin pump.
5. Remember to change the time on your pump if you will be crossing time zones.
6.
Ge
t a letter from your doctor explaining what to do for your diabetes, listing medications
and devices that you may use. The letter should also state any food or medication allergies
you may have. Also get a prescription to carry with you for any medications you may need.
Know the name and number of an endocrinologist in the area where you’re traveling may
prove useful.
7. Ca
rry bottles of insulin IN THEIR BOXES with your name, doctor’s name, your
pharmacy’s name, and medication on a pre-printed label.
8.
Co
ntact your airline for any specifics
— d
ifferent airlines have different rules regarding
diabetes supplies — don’t be surprised!
9.
Th
e pump can be worn through the scanner at the airport without causing it harm.
Don’t call attention to it.
HOSPITALIZATIONS
1. Remove pump for X-rays, MRIs.
2. Be prepared beforehand — carry a letter from your endocrinologist with orders for you to
keep the pump on, check your own glucose levels and do your own adjustments.
3. If you are unable to care for the pump, have a family member do so. If you have no family
with you, the pump may be removed, but ONLY after the nurses have orders for insulin
coverage. DKA can occur much faster after disconnecting from the pump because there is
no long-acting insulin on board.
4. The pump gives better control during and after surgery, so ask doctors to allow that it stay
connected. As soon as possible after surgery, ask to have the pump reconnected if it was
discontinued during the surgery.
5. Pregnant patients will need to move insertion site to the thigh area immediately
after beginning labor and leave the pump connected during labor. Insulin resistance
dramatically decreases after the placenta is delivered — so be prepared to decrease basal
rates. Basal rates will remain lower if the mother is breast feeding also.

30 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
STANDARDS OF CARE:
DIA
BETES
EDUCAT
ION AND
MAN
AGEMENT PROGRAM
Insulin Pump Education: Up to 8 Visits
A. Initial visit/s prior to pump start, CDE:
1. Data
collection & review; assessment of self-management skills, readiness to learn and barriers
to learning
2. Pr
erequisites for successful pumping:
a. One month of multiple injection therapy with Lantus and Humalog or Novolog
b. Many BGs showing testing at least 4 times a day for one month
c. Knowledge of pump function through watching video or doing on online pump program
3. Intr
o to pumps; basal & bolus rates, insertion sites
4. Refer
to RD for dietary counseling and CHO counting assessment
5. Assess glucose
meter skills
6. Resources: videos, books, pamphlets, web sites
7. Goal setting
B. Initial visit/s prior to pump start, RD:
1. Data collection & review; weight, food record
2. Review of meal planning and CHO counting
3. Validate ability to count carbs at home, at work or school, at restaurants and
fast-food locations
4. Goal setting
C. Follow-up visit, day of pump start, CDE (3–4 hours):
1. Pump specifics; buttons, syringe filling, priming, insertion technique
2. Initial settings
3. Pr
oblem solving, alarms
4. Restocking
supplies
5. Hypogly
cemia and hyperglycemia management, DKA prevention
6. Revie
w of tasks and follow-up plan
7. Status
of goals and reinforcement of positive changes
8. Resour
ces: videos, books, pamphlets, web sites
9. Goal setting

31 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
D. The CDE will emphasize that regulating basal and bolus rates and determining insulin to carb
ratios is essential until the blood sugars are within the preset goal ranges. Telephone support for
emergencies is available 24 hours per day.
E. Follow up visit, within one month or more frequently if needed, CDE:
1. Data
collection & review; blood sugar trends, meter download
2. Revie
w basal & bolus rates
3. Revie
w of site adequacy & insertion technique
4. Confirm completion of basal rate testing
5. Sick
day management/DKA prevention.
6. Status of goals and reinforcement of positive changes
7. Goal
setting
F. Follow-up visits with RD as needed.
1. Data
collection & review; blood sugar trends, food records
2. Review of meal plan and carb counting
3. Review of food adjustments for sick days and exercise
4. Status
of goals and reinforcement of positive changes
5. Goal
setting
G. Follow-up visits (quarterly for first year then annually) with CDE:
1. Data
collection & review; blood sugar trends, A1c results
2. Self-management
review and problem solving
3. Status
of goals and reinforcement of positive changes
4. Goal
setting
5. If child, movement toward independence in diabetes care

32 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
INSULIN PUMP FOLLOW-UP
Patient Name
____________________________________________
Date
___________________
Certified Pump Trainer
___________________________________________________________
Pump Model
______________________________________
Serial #
_______________________
BASIC REVIEW SITE CHANGE PROTOCOL
ADDITIONAL FEATURES INSTRUCTED: NOTES
BLOOD GLUCOSE RECORD
DATE TIME BG CHO GRAMS INSULIN

33 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Basal Rate Changes:
From 12 Midnight to
____________________
:
__________________________
units per hour
From
___________________
to
___________________
:
___________________
units per hour
From
___________________
to
___________________
:
___________________
units per hour
From
___________________
to
___________________
:
___________________
units per hour
Pump Trainer Signature
__________________________________
Date
____________________
INSULIN PUMP CONTACTS
Trainer:
_______________________________________________________________________
Phone:
_______________________________________________________________________
Alternate trainer:
_________________________________________________________________
Alternate phone:
_________________________________________________________________

34 of 34– Insulin Pump Therapy See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
REFERENCES
Bode, BW. Pumping Protocol A Physician’s Guide to Insulin Pump Therapy Initiation, Atlanta Diabetes
Associates, Atlanta, Georgia
Bolderman, KM. Putting Your Patients on the Pump, American Diabetes Association, 2002
Alexandria, Virginia. ISBN 1-58040-148-1
Frederickson, L, ed. In. The Insulin Pump Therapy Book Insights From The Experts. MiniMed
Technologies. Los Angeles, California. 1995 ISBN 0-9647837-0-3
Walsh, J & Roberts, R. Pumping Insulin Everything You Need To Know For Success With An Insulin
Pump, 3rd edition. ISBN 1-88480484-5
Amrhein, James A. MD and Hess, B, RN, BSN, CDE. Optimizing Glycemic Control with Diabetes
Technology. AADE 29th Annual Meeting, August 7, 2002, Philadelphia, Pennsylvania.
Brooks, AM, RN, CDE. St. Marks Hospital Diabetes Center, Salt Lake City, Utah and Kulkarni,
K, MS, RD, BC-ADM, CDE, St. Marks Hospital Diabetes Center, Salt Lake City, Utah. Core
Curriculum for Diabetes Education, Fourth Edition. Diabetes Management Therapies, Chapter 6,
pg. 203-225.

Diabetic Foot Screen*
*performed every primary care visit (for complete foot exam details, see page 2 of 4)
NO YES
Acute swelling and/or Acute deformity ...........................................................................
Skin breakdown (ulcer)
.......................................................................................................
C
allus – with deeper color changes
....................................................................................
D
igital Deformity ................................................................................................................
or chronic midfoot/rearfoot prominence
History of amputation and/or ulceration
..............................................................................
D
ystrophic Nails &/or Dry Skin
........................................................................................
N
europathy: using 10-gram nylon monofilament ................................................................
performed yearly
4 out of 10 sites imperceptible = “yes”
Resources & References:
1.
I
nt
ernational
Con
sensus on the
Dia
betic Foot, 2003.
Int
ernational Working Group on the
Dia
betic Foot (consultative section of the
Int
ernational
Dia
betes Federation)
2.
Univ
ersity of
Texa
s Health
Sci
ence
Cen
ter-
San
Antonio
Texa
s-
Depa
rtment of
Ort
hopedics-
Divis
ion of
Pod
iatry
3. Scott & White Clinic / Texas A&M University System Health Science Center-Department of Surgery, Division of Podiatry
4.
Am
erican
Dia
betes Association:
Cl
inical
Pra
ctice
Re
commendations. Diabetes Care. 2004; 27[
S1]
:63-64.
Page 4–A
Page 4–C
Page 4–B
Page 3–C
Page 3
Page 3–D
Page 3–B
Right: Dorsalis Pedis ......................................
Posterior Tibialis ..................................
Left: Dorsalis Pedis ......................................
Posterior Tibialis ..................................
PALPABLE
NONPAL
PABLE
Ankle
Brachial Index
(ABI)
Page
1–A
Assign Risk Category:
No Present Risk
____
0 No loss of protective sensation, no deformity.
Im
pending Risk
____
1 No loss of protective sensation. Deformity present.
High
Risk
____
2 Loss of Protective sensation with or without weakness,
deformity, callus, pre-ulcer or history of ulceration.
Adapted from the National Foot Treatment Center LEAP Program
FOOT PULSES:
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 04-23-04Stock #45-12000
1 of 4 – Diabetic Foot Screen – Approved 04/23/04

Diabetic Foot Exam**
**Performed Initially at Diagnosis, Annually in Primary Care
Foot History
1. Ulcers: location, time to heal, wound care necessary for healing
2.
In
fections: type, bacteria involved, medical treatment necessary
3.
Am
putations: type, time to heal, modalities used in healing process
4.
Su
rgeries/Injuries: type, location
Foot Exam
Vascular (Vasc)
1. Palpate DP, PT pulses (present or absent)
2.
Tem
perature gradient: from ankle to toes, focal “hot spots”
3.
Ge
neral Color: pink, palor, rubor on dependency
4.
Di
gital Capillary refill time: in seconds
5. ABI: for both DP & PT arteries (abnl if <0.85–0.9)
Neurologic (Neuro)
1. 10-gram nylon monofilament: test sites on feet as indicated on page 1
2.
Vi
bratory perception: via 128 Hz tuning fork (>10 secs)
O
R
Bio
thesiometer
(>25 volts)—tested at hallux
3.
Ta
ctile sensation (light touch): via cotton wool (dorsum of foot)
4.
Re
flexes: Achilles tendon
Dermatologic
(Derm)
1. General skin turgor/texture
2.
Fo
cal lesions: calluses (debride to fully assess), cracks, pigmentation
3.
In
terdigital: calluses, maceration
4. Nails: incurvated, nail plate thickness, coloration, inappropriate self-care
Musculoskeletal
(Msk)
1. General Range of Motion: ankle, subtalar, midtarsal, metarsophalangeal
2.
Fo
ot type: rectus, pes planus, pes cavus,
Char
cot foot
3. Digits: hammertoes, claw toes, mallet toes, bunion/hallux abductovalgus
4. Bony prominences
Footwear
1. Type
2. Wear pattern: outsole and upper counter distortion
3. Insole inspection: foreign bodies, staining, excessive wear
4. Socks: foreign bodies, staining, excessive wear
Social
1. Tobacco/alcohol/drug use
2. Work environment/foot demands/footwear requirements
3. Physical activities: footwear used
4. Family support: marital status, spouse/family involvement in health
5. Education: diabetes self-management
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 04-23-04Stock #45-12000
2 of 4 – Diabetic Foot Exam – Approved 04/23/04

Diabetic Foot Care/
Referral Algorithm
Complete Diabetic Foot Exam** (see page 2 of 4)
ABBREVIATIONS:
MD
med
ical doctor
DO
doc
tor of osteopathy
DPM
do
ctor of podiatric
medicine (
Pod
iatrist)
NL normal
ABNL
a
bnormal
ABI
an
kle/brachial index
TCPO2
tr
anscutaneous oxygen
pressure
NCV
nerv
e conduction
velocities
PSSD
pr
essure specified sensory
device
Normal (NL) Exam
Abnormal (ABNL)
NL vasc
NL n
euro
NL m
sk
NL derm
ABNL VASC
NL n
euro
NL m
sk
NL derm
A
REPEAT Diabetic Foot Screen*
per MD, DO, physician extender visits or DPM exam
REPEAT EVERY VISIT
NL vasc
ABNL
NEURO
NL m
sk
NL derm
B
NL vasc
NL n
euro
ABNL MSK
NL derm
C
NL vasc
NL n
euro
NL m
sk
ABNL DERM
D
DM FOOT EDUCATION
· patient/family (Diabetes Self-Management
Education)
· verbal/written
· websites
· clinic phone numbers
MD/DO/DPM (or physician extender)
VASCULAR CONSULT/TESTING
Consider: PVR, Seg. pres., ABI, TCPO2
· peripheral arteriogram as indicated
· intervention as indicated to re-establish
blood flow
NEURO or PM&R CONSULT
Consider: NCV, PSSD
· other causes: consider and rule out as
indicated
· if painful consider pharmaceutical vs.
surgical treatment
COMPLETE BIOMECHANICAL EXAM
(Podiatrist, Orthopedist)
· discuss clinical significance
· Treatment options: surgical, non-surgical
Dystrophic (thick, discolored) toenails
· EDUCATION: signs/symptoms
· HIGH RISK foot status
· Foot screen every MD/DO/DPM, or
physician extender visit
· EDUCATION: signs/symptoms
· HIGH RISK foot status
· Foot screen every MD/DO/DPM, or
physician extender visit, PRN
· EDUCATION: signs/symptoms
· Intervention: surgery→healed = low risk
· Biomech: shoes, orthoses, phys.medicine
· Follow up DPM per modality needed
· Foot screen: every MD/DO/
physician extender visit
· Debride/reduce
· Culture as needed
· Educate on condition management
· Referral as needed (podiatrist,
dermatologist)
Documentation of
vascular disease
OR
Pos
t intervention
with improvement
NORMAL testing-repeat per change in exam or onset symptoms
Dry skin, fissures
· Diagnostic tests as indicated, e.g., for fungus
· Topicals as indicated
· Referral as indicated
Ingrown toenail
· Instruct on proper nail care
· Matrixectomy if NL vasc exam
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 04/23/04
3 of 4 – Diabetic Foot Care/Referral Algorithm – Approved 04/23/04

High Risk Scenario
and Ulcer Management
Grade Ulcer
1. Assess size, depth,
tissue levels
2. X-ray exam
Peripheral Sensory
Neuropathy
& Unilateral Swelling /
calor
·
x-
ray exam
·
r/
o infection
Deep
venous thrombosis
(
DVT)
A
No skin breakdown
or
Lesion
,
no erythema
Extremely High Probability of
Charcot Arthropathy
Treat as such until proven otherwise
COMPLETE OFF-LOADING OF
EXTREMITY to prevent severe foot/
ankle deformity
Skin Breakdown Treat as Ulcer 4-C (below)
CAUTION
Consider Double ETIOLOGY,
OFF-LOAD to prevent severe foot/
ankle deformity
Hyperkeratosis
With underlying
sub-epidermal
hemorrhage
(no ulceration)
B
Follow pathways for associated abnl VASC, NEURO as indicated
1. DEBRIDE callus
2. Re-examine MSK exam for
underlying cause – follow 3-C
1. OFF-LOAD as needed (change
insole, offloading devices as
indicated)
2. Assess Footwear & Insoles
for causes, prevention
1. Re-examine/debride q 3–7 days
until skin normalized
2. Progress back to normal activities/
footwear based on etiology & risk
factors
ULCER
·
as
sess/document
C
Once healed = patient remains extremely HIGH RISK—frequent foot exams/education
Immediate Debridement
& Wound Care
TYPE of Ulcer
· underlying etiology
INFECTION
Assess: fever, WBC, ESR,
erythema, calor, drainage,
necrosis, foreign material
1. Local wound care, dressings per etiology and
clinical course
2. Surgical (OR) treatment if indicated
1. Frequent re-assessment & re-debridements as indicated
2. Continued changes in dressings/wound care
3. Advanced wound care if needed
Superficial full thickness
· not penetrating deeper
than dermis
Grade 1
Deep ulcer (below dermis)
· subcutaneous structures
(fascia, muscle, tendon)
Grade 2
All subsequent layers involved
· including bone and/or joint
· assess probing to bone/soft tissue tracts
Grade 3
1. Neuropathic
2. Ischemic
3. Neuro-Ischemic
Assess/manage causal
pathway(s) 3–A, B, C
OFF-LOAD (relieve pressure)
· non-weightbearing essential
· crutches, walkers, modified shoes/insoles,
total contact cast, etc.
1. Inflammatory response may be mitigated by diabetic complications
2. Outpatient vs. inpatient based on severity of infection & co-morbidity management
Culture & Sensitivity via
· tissue at wound base
· aspirating pus
· swab base of wound after debridement
· bone culture if suspect osteomyelitis
· blood if systemic toxicity suspected
Etiologic Agents
· Aerobic gram positive cocci most
frequent (staphylococcus)
· Gram negative & anaerobes
usually part of polymicrobial,
chronic necrotic ulcers
Antibiotics – consider:
· local institutional and community
susceptibility data when
prescribing
· published efficacy data
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 04-23-04Stock #45-12000
4 of 4 – High Risk Scenario and Ulcer Management – Approved 04/23/04

Foot Screening
Mapping Examples
Approved 04/23/04Stock #45-12000
1 of 1 – Foot Screening Mapping Examples – Approved 04/23/04 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
sUPPlement

Recommendations for Treatment of Painful
Peripheral Diabetic Neuropathy in Adults
Glycemic control
goals should be met,
if possible, prior
to the start of pain
medications
Duloxetine
1
Pros:
u
May also treat depression
Cons:
u
May cause nausea, dizzy/drowsy
u
Use with caution with other
antidepression medication
Minimum Effective Dose
60 mg daily
Gabapentin
1
Pros:
u
Generic
Cons:
u
Saturable absorption gives lower
absorption with increasing doses
u
Example: absorption at 900mg/day:
60%
3600mg/day: 33%
u
Some risk of dizzy/drowsiness/
weight gain
u
Renal adjustment of dose may be
needed
Minimum Effective Dose
100-600 mg tid
Pregabalin
1
Pros:
u
No saturable absorption issues as
with gabapentin
Cons:
u
Similar mechanism of action to
gabapentin
u
Some risk of dizzy/drowsiness/
weight gain
u
Renal adjustment of dose may be
needed
Minimum Effective Dose
50 mg tid or 150 mg hs
Tramadol
1
Pros:
u
Generic
Cons:
u
Nausea
u
Dizziness
Cautions
u
Contraindicated in known seizure
disorder or with MA
O Inh
ibitors
u
Caution with use with other
serotonergic agents
u
Avoid abrupt withdrawal
Minimum Effective Dose
50 mg bid
Tricyclic antidepressants
1
(TCA’s)
Pros:
u
Generic
Cons:
u
Anticholinergic side effects
Cautions
u
Caution with use with other
antidepressants
u
Dose-related QTc prolongation
u
Caution with other medications that
inhibit
CYP45
0 significantly
Minimum Effective Dose
12.5-50 mg at bedtime
At least
2 months
No treatment has been shown to result in superior
pain control compared to another agent
Choice of agent should be based on:
u
Side effects
u
Comorbidities
u
Cost
u
Concomitant Medications
u
Realistic expectations: Goal pain relief /partial relief
Evaluate for and treat secondary causes of peripheral neuropathy:
u
Glucose control
u
Macrocytic anemia, B12, Folic acid or Vitamin D deficiency
u
Lifestyle changes-alcohol & smoking cessation
u
Radiculopathy
u
Electrophysiology assessment recommended if glucose control does not improve pain due to
other potential etiologies
Other therapeutic agents with reported efficacy:
Topi
cal capsaicin, topical lidocaine, venlafaxine, bupropion, opioid
derivatives, alpha-lipoic acid, M
IR
E
ther
apy (Anodyne);
Consider surgical intervention if other modalities fail.
Medications Listed Alphabetically
1
Refer to prescribing information for titration recommendations Argoff CE et al. Mayo Clin. Proc. 2006 Apr; 81(4 Suppl): S12-25.
Select any of the
agents to initiate at
low dose and titrate
to minimal effective
dose
1
Change to a different
agent if initial therapy
is not effective
u
Refer to Specialist
u
Consider low dose combination therapy if partial pain relief with
any agent
u
Consider other therapeutic agents with reported efficacy
u
Consider surgical intervention/referral if other modalities fail
OR
at minimum
effective dose
1 of 1 – Recommendations for Treatment of Painful Peripheral Diabetic Neuropathy in Adults – Approved 4/26/07 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms
Approved 04/26/07Publication #45-12613

Diabetes treatment algorithms
Diabetes continues to be a disease that disproportionately affects the elderly. In Texas, approximately
16.3% of people over age 65 have been diagnosed with diabetes, compared to approximately 8.1%
of the overall population (BRFSS, 2003). Older adults with diabetes are more likely to experience
complications from diabetes, thus, elderly patients with diabetes generate most of the costs of treating
complications.
In particular the goals for treatment of the elderly person with diabetes should include:
1. Impr
oving or maintaining health and functional status of the elderly with diabetes by
maximizing glucose control.
2. Early detection and treatment of the complications of diabetes through organized, pro-active
screening efforts.
3. Aggressiv
e treatment of co-morbid risk factors, specifically hypertension and dyslipidemia.
4. Careful
monitoring of therapy to avoid common problems in the elderly: polypharmacy,
adverse drug events and inappropriate medication use.
Given that these goals are similar to those for treatment of diabetes in any age group, the patient’s stage
in the disease process and their co-morbid conditions rather than age alone are most important in
determining the appropriate course of treatment. The Council supports the basic recommendations
summarized in the Minimum Practice Recommendations flow sheet with modifications that consider
issues for elderly populations.
Health care providers and payers, including managed care organizations, should adopt the Texas
Diabetes Council’s Minimum Practice Recommendations as the basis for managing diabetes in elderly
patients.
Clinicians should strive to achieve the same levels of glycemic control (blood glucose, A1c), blood
pressure and lipid control in elderly patients with diabetes as in younger ones. Targets may be
modified in light of advanced complications, life-limiting co-morbid illness, or severe cognitive or
functional impairments.
Given the high risk of secondary complications among elderly patients with diabetes, such as
cardiovascular disease and lower extremity complications, clinicians should screen aggressively for
and treat secondary complications.
Foot screening conducted at every visit includes not only visual inspection for lesions, infections, and
calluses, but also assessment of pulses and use of monofilaments to further screen for neuropathy.
At each office visit, the clinician should specifically inquire about and consider comorbidities and
the risks associated with polypharmacy, common problems in the elderly. Increased attention may
be necessary in selecting and monitoring drug therapy in the elderly; for example, metformin may
be contraindicated because of renal disease or heart failure.
1 of 6 – Considerations for Elderly Persons with Diabetes
Considerations for Elderly Persons
with Diabetes
sUPPlement
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
Diabetes self-management education for the elderly should take into account special
instructional needs:
A) Elderly patients should be encouraged to include their caregiver or a family member in all
educational sessions
B) Educational
materials and methods should consider vision impairment, mobility, dexterity,
mental state, functional status, and financial resources.
C) Elderly
patients should be educated about possible effects of multiple medications and how
concurrent illnesses may affect their treatment, self-care, and disease progression.
D) Pr
eventing long-term complications of diabetes should be stressed.
Physiologic Changes in Glucose Metabolism
The elderly are prone to glucose intolerance and thus are at higher risk for developing diabetes.
Fasting plasma glucose increases 1–2 mg/dl and the 2-hour postprandial glucose increases on average
8–20 mg/dl per decade of age after the age of 30–40 years. The changes to glucose intolerance have
been attributed to age-related defects, post receptor defects in insulin action with decrease in velocity
of glucose transport and/or other post receptor defects. There is also a depletion of intracellular pool
of transporters or a defect in insulin-mediated translocation to the plasma membrane, along with
impairment of the intracellular glucose metabolism beyond the defect in transporters.
Diagnostic Criteria and Goals
The diagnostic criteria and goals of therapy remain the same throughout the lifespan.
u
Maintain quality of life by minimizing impacts of this disease
u
Preserve functional capacity by preventing complications
u
Minimize risk of hypoglycemia
u
Meet realistic weight goals
u
Avoid glucose readings > 200mg/dl
u
For frail elderly, aim for fasting or bedtime glucose > 100mg/dl
u
Safety precautions are imperative to prevent falls
Acute Complications are common in the Elderly
u
Increased frequency of infections (respiratory, skin, urinary)/ Foot infections can lead to
amputations
u
Difficulty healing of breaks in the skin even without infection
u
Hyperglycemic Hyperosmolar Nonketotic Syndrome
u
DKA, not rare
u
Hypoglycemia related to sulfonylurea or insulin treatment, especially with declining renal
function
2 of 6 – Considerations for Elderly Persons with Diabetes See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
Atypical Presentation of Hyperglycemia in the Elderly
u
A vague sense of not feeling oneself.
u
Electrolyte imbalance and dehydration (blunted sense of thirst).
u
Incontinence (masking polyuria).
u
Appetite loss (due to depression, GI disease, or drug side effects).
u
Fatigue (“just getting old”) and gradual profound loss (unnoticed for months).
Diabetes Symptoms
Often Pr
esent Differently in Frail
Elderly
PATHOPHYSIOLOGIC STATE
TYPICAL
PRESENTATION
COMMON PRESENTATION
IN FRAIL ELDERLY
Hyperglycemia/
hyperosmolarity
Polydipsia Impaired vision, confusion,
dehydration
Catabolism due to lack of insulin Polyphagia Weight loss, anorexia
Increased urinary volume due to glucosuria Polyuria Incontinence
Drugs That May Worsen Hyperglycemia in the Elderly
u
Glucocorticoids
u
Thiazide diuretics
u
Phenytoin
u
Lithium and Phenothiazines
u
Estrogens
u
Growth Hormone
u
Isoniazid and Sympathomimetic agents
u
Sugar-containing medications
Altered Presentation of Hypoglycemia in the Elderly
u
Adrenergic symptoms: sweating, nervousness, tremor
u
Neuroglycopenic symptoms: confusion
u
Elderly lose the adrenergic symptoms (loss of autonomic nerve function) and have more
profound neuroglycopenic symptoms than the young: reversible hemiparesis.
u
This occurs late in the course of hypoglycemia.
Consequences of Severe Hypoglycemia:
u
Tissue damage in elderly patients with impaired cardiac and cerebral circulation and
serious chronic neurological consequences
3 of 6 – Considerations for Elderly Persons with Diabetes See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
u
Exacerbation of ischemic heart disease with anginal symptoms
u
Injuries including fractures
u
Death caused by hypoglycemia or its consequences
Cause of Serious or Fatal Hypoglycemia
u
Skipping meals or not eating enough
u
Error in dosage of sulfonylurea or insulin agents (10% of SFU-related hypoglycemia
patients die)
u
Excessive activity or exercising with a low blood sugar
u
Alcohol abuse associated with skipped meals
Contraindications of Tight Control in the Elderly
u
Dementia
u
Autonomic nerve dysfunction
u
Physical disability
u
Social isolation or food restriction
u
Chronic renal insufficiency
u
Cirrhosis
Goal: Decrease hyperglycemic symptoms and prevent hyperosmolar state
Monitoring in the Elderly
u
Most elderly incorrectly perform glucose and urine tests.
u
Blood glucose monitoring correlates to A1c and is a better tool for titrating insulin.
u
Assess albuminuria to assess cardiovascular status and treat HTN/Lipids.
u
Feet should be screened/treated vigorously.
Medical Nutrition Therapy Goals and Points of Consideration
u
Individualize dietary modifications. Consider preferences and household.
u
Minimize unnecessary restrictions.
u
Vitamin and mineral supplements may be indicated. Talk to physician prior to starting
any supplement.
u
Minimal weight loss for obese can be very effective. Limit intake of saturated and trans
fatsasmuchaspossible.Saturatedfatshouldconsistoflessthan7%ofthetotalcalories*.
u
Unless medically contradicted, encourage drinking 2 quarts of water per day.
*DiabetesCare,2007Jan;30Suppl1S11
4 of 6 – Considerations for Elderly Persons with Diabetes See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
u
Recommend at least 20 grams of fiber per day to prevent constipation and reduce heart
disease and cancer.
u
Calcium intake should be encouraged. Those older than 70 years need 1,200 mg per day
(32 ounces of milk equivalent).
u
The recommended daily dose of Vitamin D and B-12 supplements for those over the age
of 70 are 600 IU for Vitamin D and 2.4 micrograms for Vitamin B-12 (many elderly are
unable to absorb Vitamin B-12 from food).
u
Overdose of Vitamin A is more likely in the elderly, since Vitamin A is absorbed more
readily and clears more slowly.
u
Protein needs to make up greater part of elders’ meal plans since they usually take in
fewer calories.
Exercise in Older Adults
u
Consider risks and benefits of specific activities.
u
Conduct pre-exercise evaluation (medical evaluation, ECG, exercise stress testing).
u
Start with low intensity; slowly increase activity.
u
Range-of-motion exercises, walking and swimming are great choices.
u
Perform some light weight lifting (strength building).
Diabetes-Associated Changes That Affect Teaching-Learning
u
Sensory — (visual acuity, lens clarity, night vision, hearing)
u
Impaired seeing syringe marks, perceiving blue-tone colors, interpreting home glucose
monitoring instruments
u
Impaired communication may lead to non-adherence
u
Cognition — memory, complex psychomotor tasks
u
May need repetition or caretaker assistance
u
May have difficulty with insulin administration (mixing insulins and injection, site
rotation) and glucose monitoring
u
Cutaneous — skin vibratory and thermal sensitivity, tactile sensitivity
u
Impaired ability to discern temperature and pressure
u
otential for unawareness of burns and ischemia
u
Decreased manual dexterity for injections and glucose monitoring
u
Urinary — decreased renal function, altered renal threshold for glucose
u
Potential for hypoglycemia, increasing drug half-life
u
Decreased utility of urine testing
5 of 6 – Considerations for Elderly Persons with Diabetes See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
u
Gustatory, Olfactory — taste, smell
u
Reduced dietary adherence
u
Gastointestinal — thirst mechanism, motility, delayed gastric emptying
u
Altered dietary intake
u
Potential for hypoglycemia and dehydration
u
Vestibular-Proprioceptive-Equilibrium — sense of bodily orientation
u
Vertigo and imbalance, potential for falls
u
Decreased motivation for exercise/activity
u
Limit other medications that can increase risk of falls:
u
Drowsiness
u
Dizziness
u
Urinary or fecal problems
6 of 6 – Considerations for Elderly Persons with Diabetes See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
Introduction
High Risk for Diabetes-related Complications
The elderly in long-term care facilities such as nursing homes or assisted living centers are at high risk
for developing diabetes-related complications such as infections, non-healing wounds, amputations,
myocardial infarction, strokes, and particularly, electrolyte depletion and dehydration that lead to
high hospitalization rates in this population.
The elderly are often unable to detect and report problems due to age-related factors such as decreased
cognition, sensation, mobility, communication, thirst response, that are typically associated with
aging. Diabetes-related complications appear differently in the elderly, especially the frail. Often
symptoms such as urinary frequency, nocturia or incontinence, volume depletion or dehydration,
excessive skin alterations (ulcers), infections, or delayed wound healing, dental caries, periodontal
disease, burning mouth, foot ulcers or deformities, and increased pain perception, rapid weight
alteration, urinary frequency are symptoms that can be attributed to the aging process or noted as
insignificant are often not associated with symptoms of complications secondary to diabetes.
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Adapted from American Medical Directors
Association ( 2002), British Diabetic Association
Report (1999) & Pandya, AMDA Clinical Practice
Guidelines Steering Committee
Evaluation
Diabetes-Related of
Complications
Glycemic Control Pre-prandial and post-prandial glucose levels, A1c
Assess Cardiovascular Disease Risk Factors
or Conditions
Assess and treat atherosclerotic heart &
cerebrovascular disease and/or cardiovascular
complications
Order electrocardiogram, echocardiogram, chest
X-ray, arterial doppler studies of the legs, cognitive
testing, computed tomography (CT), and brain
magnetic resonance imaging (MRI)
Consider prescribing: enteric-coated aspirin,
clopidogrel or aspirin/extended release dipyridamole,
beta-blockers
Guidelines for Management of the Elderly
with Diabetes in Long-Term Care Facilities
1 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities
sUPPlement
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Assess Peripheral or Autonomic
Neuropathy
Foot deformity, gait impairment
Psychological Assessment Unrecognized depression, cognitive impairment
Determine Severity of Complications CBC, basic serum chemistry, renal and hepatic
function, careful review of facility glucose logs (Not
necessary to do A1c for treatment regimen change)
Obtain History Recent hospital records, community physicians &
family members
Health Care
Provider
Guidelines to notify health care provider
should be established within institution
and for patient
Glucose <60mg/dl or <75mg/dl with symptomatology
of hypoglycemia (See “Hypoglycemia” in Diabetes
Tool K it)
Marked changes in glucose: If >250mg/dl along with
change in status, condition
Glucose >300mg/dl for 3 consecutive days (Unless
represents improvement to status or orders note
method of management)
Difficulty with oral intake for > 2 days or more
accompanied with fever, lethargy, abdominal pain,
hypotension, respiratory distress, etc.
2 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
See Algorithms:
Glycemic Control for Type 2 Diabetes in
Children & Adults
Insulin for
Typ
e 1 Diabetes in Children
and Adults
Insulin for
Typ
e 2 Diabetes in Children
and Adults
Initiation of Insulin
The
rapy for
Typ
e
2 Diabetes in Children and Adults: A
Simplified Approach
IV Insulin Infusion Protocol for Critically
Ill Adult Patients in the ICU Setting
ICU Insulin
Ord
ers
Insulin Pump
The
rapy
Anti-Diabetes Agents
Metformin:
Consider if obese, not recommended over age 80, use
only with normal liver, renal function, do not use with
CHF, acute illness
Secretagogues, Sulfonylureas:
Consider for non-obese or mildly obese
Consider for insulin resistance, obese patient
Thi
azolidinediones:
Not used with Class III, IV CHF
Normal liver function
Alpha-Glucosidase Inhibitors:
For patients near A1c goal (milder diabetes) and/or
post-prandial hyperglycemia
Incretins:
No information at this time for use in the elderly
population
3 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Prevention &
Treatment of
Complications
Hypoglycemia
(See “Hypoglycemia,” Section, 8.1, in
Diabetes
Too
l Kit,
TDC
)
Eld
erly (particularly frail) may exhibit
atypical symptoms of hypoglycemia such
as: disorientation, incoordination, altered
personality, falls for unknown cause.
Morbidity is heightened with
nocturnal hypoglycemia, cognitive and
communication problems, chronic cardiac
and liver disease, and adrenal or pituitary
insufficiency.
Treat with carbohydrate in the form of glucose,
sucrose tablet or juice combined with light snack
containing protein: Oral glucose paste, intramuscular
glucagon, intravenous 50% dextrose
Consider and assess for risks of hypoglycemia
High doses, rapid acting insulin (with delayed meal
consumption)
Inconsistent calorie intake, hypoglycemia
unawareness
Insulin & Hypoglycemia:
To decrease risk of hypoglycemia:
Avoid prolonged use of “sliding-scale insulin”
(graded increases in short- or rapid-acting insulin for
every 50 to 100 mg/dl rise in blood glucose, usually
administered before meals and at bedtime); increases
morbidity, nursing time, not shown to improve
metabolic control
Sliding scale should be reserved for short-term glucose
control post illness or surgery
Fixed daily doses of insulin are recommended once
daily insulin requirements are noted
Endocrinologist consultant recommended for labile
diabetes or for those on insulin pump
4 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Foot Care
See Foot Care Materials:
Foot Screening Mapping
Exa
mples
Diabetic Foot Screen
Diabetic Foot
Exa
m
Diabetic Foot Care/Referral
High Risk Scenario & Ulcer Management
Recommendations for
Tre
atment of
Painful Peripheral Diabetic Neuropathy
Assess skin and soft-tissue for alterations, sensation,
color, temperature, circulation, presence of
neuropathy, foot deformity, gait
Order protective footwear with accommodating
insoles
Assure that feet are examined during all scheduled
visits
Teach preventive foot care to patients, families,
nursing assistants
If foot at risk: Order Routine Podiatric care; daily foot
care by patient and caregivers
With mild infection or ulcer consider local dressings;
baseline X-ray for bone integrity or osteomyelitis;
podiatry or wound care referral as needed (so that
wounds are treated, reassessed, and debrided on site if
at all possible).
Limb-threatening ulcer or infection: consider
hospitalization; referral to podiatry or vascular surgery
5 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Eye Disease
(See Chronic complications sections in
Diabetes
Too
l Kit,
TDC
, Section, 9.1)
Or
al care
See Diabetes and Gum Disease, H9.13
Hypertension
See Algorithm: Hypertension for Diabetes
in Adults
Diabetic nephropathy
Dyslipidemia
See Algorithm: Lipid
Tre
atment for
Typ
e 1
and
Typ
e 2 Diabetes in Adults
Macrovascular Risk Reduction in
Diabetes: Antiplatelet
The
rapy
Assessment of pain, infections, visual disturbance
Annual dilated eye examination if appropriate
Diabetes, hypertension, and proteinuria control
prevention
Evaluate oral cavity for pain, signs of infection, eating,
swallowing disorders.
Consider dietitian consult, prophylactic antibiotics,
and/or dental services
Consider angiotensin-converting enzyme (ACE)
inhibitors or angiotensin receptor blockers (ARBs)
Consider dietitian & nephrologist consultation
Consider protein-restricted diet
Utilize multiple methods to control of blood glucose
and hypertension: angiotensin-converting enzyme
inhibitors, angiotensin receptor blockers
Consider dietitian consult
Important to maintain control of lipids, blood
pressure, blood glucose
Utilized lipid-lowering medication as applicable and
appropriate
Note: Dietary restriction is not recommended in frail
elderly patients
Immunization Consider influenza & pneumococcal vaccine
An Interdisciplinary
Approach
Health Care Provider Assessment and
Tea
m Intervention to evaluate functional
and medical status, and rehabilitation
needs
Team members needed include: consultations from
dietitian, pharmacist, physical therapist, activity
therapist, podiatrist, mental health professional as
needed
6 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Medical Nutrition
Therapy
Dietitian Consult & Assessment
Warranted
No “ADA” diet recommended;
Assess for common problems such as,
chewing difficulty, decreased appetite,
undernourished, anorexia, depression,
dependency, chewing difficulty, and
chronic gastrointestinal complaints.
See Algorithm:
Nutrition Recommendations and
Interventions for Diabetes
Diabetes Medical Nutrition
The
rapy &
Prevention
“No concentrated sweets”: or “no added sugar” diets
are inappropriate and do not contribute to good
outcomes (J Am Dietetic Assoc 2001;101:1463-1466)
and affect quality of life.
Avoid calorie restricted diets particularly in those
with major infections, major surgical procedures with
multiple complications incurred
Avoid fat and sugar-free restriction, except for obese
and/or dyslipidemic residents: decreases palatability
of food
Meals should be prepared considering cultural,
religious themes (Consider eating habits, food
preferences, and food brought in by family members)
Balanced meals and snacks with consistent
carbohydrate content should be consumed at
consistent times of the day
Lean meats, nuts, eggs, fish, (6–8 servings/oz., 1 oz.
Meat, fish, poultry, cheese
Low & Non Fat Milk , Yogurt (2–3 servings, 1 cup
milk, yogurt
Dark, bright colored vegetables (6 servings ½ cooked,
1 cup raw)
Dark Colored Fresh fruit (2 servings- small, size of
tennis ball)
Whole enriched fortified grains, beans and strachy
vegetables (5–6 servings)
Exercise regimens should be individualized with
attention to diabetes related complications, preventing
worsening of glycemic status, hypoglycemia and
adjusting oral medication and/or insulin therapy
according to optimize glucose and prevent
hypoglycemia
Special formulas are expensive, often unnecessary;
the health care provider should pay close attention to
glucose logs while making periodic pharmacological
regimen adjustments
7 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
GUIDELINES
FOR
DIABETES MANAGEMENT
INDIVIDUALIZE CARE ACCORDING TO:
PREFERENCES, FUNCTIONAL AND MEDICAL
STATUS, AND
PROGNOSIS OF PATIENT
Personal Care Personal hygiene, skin, oral & foot care
20-40% Have neuropathy, peripheral
vascular disease, or both
Caregivers are needed for basic daily, mobility,
toileting care to prevent ulcers or infected feet
References American Medical Directors Association. (2002).
Managing diabetes in the long-term care setting,
Columbia (MD): American Medical Directors
Association (AMDA);
Pandya, N. (2003) Long-term care guidelines for
diabetes management, Clinical Practice Guidelines
Steering Committee, Albuquerque, NM, AMDA,
Caring for the Ages, 4(2).
British Diabetic Association Report. (1999).
Guidelines of practice for residents with diabetes in
care homes, A report prepared by a Working Party
of the British Diabetic Association on behalf of the
Diabetes Care Advisory Committee.
Key Points About Diabetes in LTC
u
Diabetes management must be individualized: patients’ preferences, medical and functional
status, and prognosis should be taken into consideration.
u
Strict dietary restrictions should be replaced with a diet plan that incorporates eating habits
and food preferences.
u
Weight loss and increased activity may not be possible for many patients, and attempts to
implement this may delay proper treatment.
u
The physician is responsible for controlling blood glucose with pharmacological means if
possible, to match food consumption.
u
A thorough clinical evaluation of the patient is essential to determine the burden of diabetes
and to formulate a treatment plan.
u
An interdisciplinary effort is required to manage this complex disease.
u
Daily attention to oral care and skin care may prevent complications overall. Specifically
nutritional problems, pressure sores, foot ulcerations, and deep infections may be eliminated.
u
Patient-specific treatment goals and reasons for not following recommended treatments
should be documented in the medical record.
u
Glycemic goals should be liberalized for the patient at risk of frequent hypoglycemia and for
the patient who is at the end of life.
8 of 8 – Guidelines for Management of the Elderly with Diabetes in Long-Term Care Facilities See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Screening Recommendations for IFG, IGT & DM
FPG Annually
2
:
if above 100 mg/d
L c
onfirm with repeat fasting
glucose. Avoid OGTT if possible
2
if below 100 and high risk based on multiple
risk factors and/or metabolic syndrome consider
checking postload glucose
2
Diabetes Management
Goals of Therapy: consider comorbidities before
setting targets
1
:
A1c < 7% if attainable without significant
hypoglycemia
3
BP <130/80 mmHg
LDL <100 mg/dL (<70 if clinical vascular disease
present)
Aspirin therapy (if no contraindications-older adults
are more susceptible for G
I bl
eeds)
Smoking cessation
Cardiovascular Risk Reduction
- As
sess fasting lipids
Ref
er to
T
DC
Al
gorithm
on
Lip
id Management; use fibrates in caution due
to renal insufficiency & consider 24 hour urine for
Creat
inine
Cle
arance
- Obtain baseline EKG
-
Con
sider stress testing based on appropriate
evaluation of comorbidities & life expectancy
-
T
re
at
B
P
t
o goal
-
I
ni
tiate A
CE i
nhibitor or A
RB i
f indicated
- Aspirin therapy if no contraindication
Initial Intervention:
1)
When
considering interventions, consider the following: life expectancy, comorbidities and specific
geriatric syndromes such as cognitive impairment, history of falls, & sensory impairment
2)
Dia
betes
Ed
ucation:
Bl
ood glucose monitoring: establish daily glucose pattern (if appropriate
and patient/caregiver able) using preprandial and 2 hours postprandial glucose checks;
Lif
estyle (exercise, weight control); Medical
Nut
rition
The
rapy (
See T
DC
A
lgorithm &
Too
lkit)
3)
C
ar
diovascular
Ris
k
Red
uction [see
C
V
r
isk reduction on left below]
4)
If le
an body habitus, consider diagnosis of
Typ
e 1
DM an
d consider measuring
I
C
A & GAD
an
tibodies and
C-p
eptide.
If po
sitive antibodies or low
C-p
eptide then consider insulin therapy.
5)
Con
sider initiation of pharmacologic monotherapy at this time if A1c > 7-7.5%
[see pharmacologic therapy below]
Goals achieved:
continue therapy
A1c every 3-6 months
Not at goal within 3 months
Add second agent if on monotherapy
Not at goal within 3-6 months
Con
sider adding third agent and/or
referral to diabetes specialist
Pharmacologic Therapy
Considerations in choosing agent
1
: risk of hypoglycemia,
comorbidities, polypharmacy, cost, life expectancy
Start with monotherapy
1
: acarbose, BAR, DPP-4, incretin
mimetic, insulin, meglitinide, metformin,
S
U
, TZD
4
Footnotes:
1
Chr
onologic and physiologic age may diverge after age 65 so patients need to be assessed individually.
The p
resence of comorbidities impacts therapeutic approach:
Lif
e expectancy,
CHF
,
Ren
al disease,
Cog
nitive impairment,
Dep
ression,
Inc
ontinence,
Inj
urious falls,
Per
sistent pain, Hip fracture, Malignancy,
Nut
ritional
Sta
tus and
Pol
ypharmacy (see
T
DC
Dia
betes
Too
lkit).
Cer
tain individuals aged < 65 years may benefit from this approach.
If a
m
ore aggressive approach is desired please see
T
DC
A
lgorithm for Glucose
Con
trol for
Typ
e 2
DM in Chi
ldren and Adults and
Dia
betes
Too
lkit.
2 Fasting may miss people who have postload hyperglycemia.
If th
e person has the Metabolic
Syn
drome with F
PG be
low 126 mg/dl, consider also obtaining a postload glucose level. For postload glucose a 2 hour postprandial
is preferred. Avoid OGTT if possible due to associated risks in this population. Postprandial glucose and/or postprandial urinalysis for glycosuria is less sensitive but have a place within certain screening programs where other
methods are not practical.
IGT i
s a 2 hour postload of 140-199 mg/d
L. DM is a 2 h
our postload of > 200 mg/d
L.
3
Con
sider an individual target of <6% if attainable without significant hypoglycemia (
Ple
ase see
T
DC
A
lgorithm for Glucose
Con
trol for
Typ
e 2
DM in Chi
ldren and Adults).
If un
able to reach <7% without hypoglycemia then target is < 8%
4
S
U
s no
t preferred due to risk of hypoglycemia; if an
S
U
i
s used then it is recommended to avoid use of glyburide and chlorpropamide.
TZDs mu
st be used with caution in people with
CAD o
r
CHF
.
Ref
er to
T
DC
Ins
ulin Algorithm for insulin use.
Glucose goals not met within 3-6 months
Geriatric is defined as age 65+ years
1
Diabetes Management
Screening and Management of Hyperglycemia
in the Geriatric Population
1 of 2 – Screening and Management of Hyperglycemia in the Geriatric Population – Approved 10/23/08
Approved 10/23/08
See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

References
1.
S
in
clair A, Finucane
P, ed
s.
Dia
betes in
Old A
ge. 2nd ed.
Chi
chester,
UK: J
ohn Wiley &
Son
s,
Ltd
; 2001.
2.
Ma
dden KM,
Ted
der G,
Loc
khart
C. The o
ral glucose tolerance test induces myocardial ischemia in healthy older adults.
Cli
nical and
Inv
estigative Medicine 2007;30(3):
E11
8-26.
3.
C
ha
ng AM,
Smi
th MJ,
Blo
em
CJ, G
alecki A
T, Ha
lter J
B. Eff
ect of lowering postprandial hyperglycemia on insulin secretion in older people with impaired glucose tolerance. Am J
Phy
siol
End
ocrinol Metab
2004;287(5):
E906-
11.
4. Meneilly GS, Cheung E, Tuokko H. Altered responses to hypoglycemia of healthy elderly people. J Clin Endocrinol Metab 1994;78(6):1341-8.
5. Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 2006;29(8):1794-9.
6.
C
ar
e
Cal
ifornia Healthcare Foundation/American Geriatrics
Soci
ety
Pan
el in
Imp
roving
Car
e for
Eld
ers with
D. Gu
idelines for
Imp
roving the
Car
e of the
Old
er
Per
son with
Dia
betes Mellitus. J Am Geriatr
Soc
2003;51(5s):265-80.
7.
Ka
takura M,
Nak
a M, Kondo
T, et a
l.
Pro
spective analysis of mortality, morbidity, and risk factors in elderly diabetic subjects:
Nag
ano study.
Dia
betes
Car
e 2003;26(3):638-44.
8.
L
ip
scombe
L
L
, Go
mes
T, Lev
esque
L
E
, Hu
x J
E, Ju
urlink
D
N
, Al
ter
DA. Thi
azolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 2007;298(22):2634-43.
9. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006;295(16):1935-40.
10.
Ku
o HK, Jones
R
N
, Mi
lberg W
P, et a
l.
Eff
ect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: a longitudinal analysis of the advanced cognitive training for independent and vital
elderly cohort. J Am Geriatr
Soc 2
005;53(7):1154-61.
11.
McBea
n AM, Huang Z,
Vir
nig
BA, Lur
ie
N, Mu
sgrave
D. Raci
al variation in the control of diabetes among elderly medicare managed care beneficiaries.
Dia
betes
Car
e 2003;26(12):3250-6.
12.
Ho
lt
RM, Schw
artz F
L, Shub
rook JH.
Dia
betes care in extended-care facilities: appropriate intensity of care?
Dia
betes
Car
e 2007;30(6):1454-8.
13. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC, Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27(3):699-703.
14.
O
ke
reke
O
I
, Ka
ng JH,
Coo
k
N
R
, et a
l.
Typ
e 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr
Soc 2
008;56(6):1028-36.
15.
T
he ADVANC
E
Col
laborative Group.
Int
ensive
Blo
od Glucose
Con
trol and
Vas
cular
Out
comes in
Pat
ients with
Typ
e 2
Dia
betes.
N Eng
l J Med 2008;358(24):2560-72.
16.
T
he A
ction to
Con
trol
Car
diovascular
Ris
k in
Dia
betes
Stu
dy G.
Eff
ects of
Int
ensive Glucose
Low
ering in
Typ
e 2
Dia
betes.
N Eng
l J Med 2008;358(24):2545-59.
17.
E
ur
opean
Dia
betes Working Group for
Old
er
Peo
ple.
Cli
nical Guidelines for
Typ
e 2
Dia
betes:
Eur
opean
Uni
on Geriatrics Medicine
Soci
ety; 2004.
Abbreviations
AG
I Alp
ha-Glucosidase
Inhib
itors
A
CE i
nhibitor
An
giotensin
Con
verting
Enz
yme
Inh
ibitor
A
RB
Ang
iotensin
Rec
eptor
Blo
cker
BAR
B
il
e Acid
Res
in (colesevelam)
CAD
C
or
onary Artery
Dis
ease
DP
P
-
4
Dip
eptidyl peptidase-4
Inh
ibitor
F
PG Fa
sting
Pla
sma Glucose
IFG Impa
ired Fasting Glucose
IGT
I
mpai
red Glucose
Tol
erance
GAD* Gl
utamic Acid
Dec
arboxylase
ICA* Isl
et
Cel
l Antibodies
OGTT
O
ra
l Glucose
Tol
erance
Tes
t
SU
S
ul
fonylurea
TZD
T
hi
azolidinedione
*note:
I
C
A an
d GA
D a
ntibodies usually take 1-2 weeks to be
reported.
If result is positive then patient has autoimmune
mediated diabetes and insulin needs to be considered and
oral agents may need to be discontinued
Hypoglycemia: Autonomic hypoglycemic warning signs may not
be recognized in older adults due to changes in counter regulatory
hormone response. Symptoms of hypoglycemia are often mistaken
for co-existing medical conditions including postural hypotension,
Parkinson’s, dementia, traumatic brain injury or CVA. Patients that
cannot communicate verbally with caregivers are at greater risk.
2 of 2 – Screening and Management of Hyperglycemia in the Geriatric Population – Approved 10/23/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
Diabetes treatment algorithms

Diabetes treatment algorithms
Minimum Standards for Diabetes Care Under Managed Care in Texas. 1995.
www.texasdiabetescouncil.org
Managed Care Work Group of the Texas Diabetes Council.
Minimum Standards for Diabetes Care in Texas. 1999.
www.texasdiabetescouncil.org
Managed Care Work Group of the Texas Diabetes Council.
Minimum Practice Recommendations Flow Sheet. 1998.
www.texasdiabetescouncil.org
Davidson J, Reasner C, Hollander P, Riley W, Falvey T, Spellman CW, Varma S, DeFronzo R, Kurtzman N, Forshay
R, Garber A, Sherman L, Fehrenkamp S, Hollander P, Varma S, Kurtzman N, Hale D, Garza-Abijaoude L, Walz B,
Plotkin R, DeNino L, Kajander J, Weiss B, et.al.
Minimum Practice Recommendations Flow Sheet. 2005.
www.texasdiabetescouncil.org
Spellman CW, Jackson J, Triplitt C, Hollander P, Sloan L, Walz B, Villagomez E, Garza-Abijaoude L, Varma, S.
Insulin Algorithm for Type 2 Diabetes Mellitus in Children and Adults. 2005.
www.texasdiabetescouncil.org
Jackson J, Spellman CW, Triplitt C, Hollander P, Sloan L, Bakdash M, Varma S, Walz B, Villagomez E, Menchaca J.
Initial Insulin Therapy for Type 2 Diabetes Mellitus in Children and Adults: A Simplified Approach. 2005.
www.texasdiabetescouncil.org
Spellman CW, Triplitt C, Jackson J, Villagomez E, Hollander P, Sloan L, Bakdash M, Varma S, Walz B, Menchaca J.
Exercise Algorithm for IFG/Type 2 Diabetes Prevention and Therapy. 2005.
www.texasdiabetescouncil.org
Walz B, Hollander P, Villagomez E, Jackson J, Spellman CW, Triplitt C, Sloan L, Garza-Abijaoude L.
Weight Loss Algorithm for Overweight and Obese Adults. 2005.
www.texasdiabetescouncil.org
Hollander P, Walz B, Villagomez E. Jackson J, Spellman CW, Triplitt C, Garza-Abijaoude L, Sloan L.
Prevention and Delay of Type 2 Diabetes in Children and Adults with IFG and/or IGT. 2005.
www.texasdiabetescouncil.org.
Hollander P, Jackson J, Spellman CW, Triplitt C, Sloan L, Bakdash M, Varma S, Menchaca J, Villagomez E, Walz B,
Garza-Abijaoude L.
Weight Management Algorithm for Overweight Children and Adolescents. 2005.
www.texasdiabetescouncil.org
Hollander P, Triplitt C, Spellman CW, Jackson J, Walz B, Menchaca J, Villagomez E, Garza-Abijaoude L, Sloan L,
Bakdash M, Varma S.
Macrovascular Risk Reduction in Diabetes: Antiplatelet Therapy 2006.
www.texasdiabetescouncil.org
Triplitt C, Jackson J, Sloan L, Spellman CW.
Glycemic Control Algorithm for Type 2 Diabetes Mellitus in Children and Adults. 2006.
www.texasdiabetescouncil.org
Jackson J, Triplitt C, Hollander P, Spellman CW, Sloan L, Bakdash M, Varma S, Villagomez E, Walz B, Menchaca J.
Insulin Algorithm for Type 1 Diabetes Mellitus in Children and Adults. 2006.
www.texasdiabetescouncil.org
Jackson J, Triplitt C, Spellman CW, Hollander P, Sloan L, Bakdash M, Varma S, Walz B, Villagomez E.
Texas Diabetes Council Authorship Minimum
Practice Recommendations, Algorithms and Reports
Revised 12/4/08
1 of 2 – Texas Diabetes Council Authorship Minimum Practice Recommendations,
Algorithms and Reports – Revised 12/4/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp

Diabetes treatment algorithms
IV Insulin Infusion Protocol for Critically Ill Adult Patients in the ICU Setting. 2006.
www.texasdiabetescouncil.org
Spellman CW, Jackson J, Triplitt C, Hollander P, Sloan L, Bakdash M, Varma S, Walz B, Villagomez E.
Lipid Treatment Algorithm for Type 1 and Type 2 Diabetes Mellitus in Adults. 2006.
www.texasdiabetescouncil.org
Wyne KL, Spellman CW, Jackson J, Triplitt C, Sloan L, Hollander P.
Medical Nutrition Algorithm IFG/Type 2 Diabetes Prevention & Therapy. 2006.
www.texasdiabetescouncil.org
Garza-Abijaoude L, Villagomez E, Walz B, Hollander P, Spellman CW, Triplitt C, Sloan L.
Diabetic Foot Care / Referral Algorithm. 2006.
www.texasdiabetescouncil.org
Murdoch DP, Jackson J, Spellman CW, Triplitt C, Hollander P, Sloan L, Bakdash M, Harkless L.
Hypertension Algorithm for Diabetes Mellitus in Adults. 2007.
www.texasdiabetescouncil.org
Triplitt C, Sloan L, Hollander P, Spellman CW.
Recommendations for Treatment of Painful Peripheral Diabetic Neuropathy. 2007.
www.texasdiabetescouncil.org.
Triplitt C, Spellman CW, Wyne KL, Greene S, Sloan L, LaFontaine J.
Glycemic Control Algorithm for Type 2 Diabetes in Children and Adults. 2007.
www.texasdiabetescouncil.org.
Spellman CW, Wyne KL, Triplitt C, Greene S, Sloan L.
IV Insulin Infusion Protocol for Critically Ill Adult Patients in the ICU Setting. 2007.
www.texasdiabetescouncil.org
Spellman CW, Triplitt C, Wyne KL, Greene S.
ICU Insulin Orders: IV Insulin Infusion Protocol. 2007.
www.texasdiabetescouncil.org
Spellman CW, Wyne KL, Triplitt C, Greene S.
Lipid Treatment Algorithm for Type 1 and Type 2 Diabetes Mellitus in Adults. 2008.
www.texasdiabetescouncil.org
Wyne KL, Triplitt C, Spellman CW, Greene S, Hollander P.
Transition Algorithm From I.V. to S.Q. Insulin For Patients With Diabetes or Hyperglycemia. 2008.
www.texasdiabetescouncil.org
Spellman CW, Greene S, Wyne KL, Hollander P, Triplitt C, Sloan L.
Orders for Adults with DKA and Hyperglycemic Hyperosmolar State (HHS). 2008.
www.texasdiabetescouncil.org
Spellman CW, Greene S, Wyne KL, Hollander P, Triplitt C, Sloan L.
Screening and Management of Hyperglycemia in the Geriatric Population
www.texasdiabetescouncil.org
Wyne KL, Spellman CW, Triplitt C, Greene S, Hollander P, Sloan L.
2 of 2 – Texas Diabetes Council Authorship Minimum Practice Recommendations,
Algorithms and Reports – Revised 12/4/08 See disclaimer at www.tdctoolkit.org/algorithms_and_guidelines.asp
