
Journal
of
Physiology
(1997),
502.3,
pp.649-659
Distribution
of
sarcomere
length
and
intracellular
calcium
in
mouse
skeletal
muscle
following
stretch-induced
injury
C.
D.
Balnave,
D.
F.
Davey
and
D.
G.
Allen
Department
of
Physiology
and
Institute
of
Biomedical
Research
(F13),
University
of
Sydney,
NSW
2006,
Australia
1.
The
effect
on
sarcomere
organization
of
stretching
intact
single
skeletal
muscle
fibres
by
50
%
of
their
optimum
length
(L.)
during
ten
consecutive
short
tetani
was
investigated.
Stretch
reduced
tetanic
force
to
36
+
4
%
of
the
pre-stretch
condition.
Sarcomere
organization
was
analysed
using
both
electron
and
confocal
microscopy.
For
confocal
microscopy
the
striation
pattern
was
examined
by
fluorescently
staining
F-actin
with
rhodamine-phalloidin.
2.
Electron
microscopy
revealed
that
fibres
which
had
been
stretched
during
contraction
contained
areas
of
severe
sarcomere
disorganization,
as
well
as
adjacent
sarcomeres
of
normal
appearance.
3.
Confocal
images
of
stretched
fibres,
which
had
been
fixed
and
stained
with
rhodamine-
phalloidin,
showed
focal
regions
of
overstretched
sarcomeres
and
regions
where
sarcomeres
of
adjacent
myofibrils
were
out
of
alignment
with
each
other.
Analysis
of
all
sarcomeres
along
the
length
of
fibres
showed
regions
of
sarcomere
inhomogeneity
were
distributed
throughout
the
fibre
length
and
cross-section.
4.
Fibres
were
microinjected
with
the
fluorescent
[Ca2+]i
indicator
fura-2
before
being
stretched.
Conventional
wide-field
fluorescence
imaging
microscopy
showed
that
the
tetanic
[Ca2+],
was
reduced
after
stretching
but
remained
uniformly
distributed.
5.
This
study
confirms
the
finding
that
stretch-induced
muscle
injury
has
components
caused
by
disorganization
of
the
myofibrillar
array
and
by
failure
of
tetanic
Ca2+
release.
The
structural
damage
is
spatially
heterogeneous
whereas
the
changes
in
Ca2+
release
appear
to
be
spatially
homogeneous.
Human
and
animal
studies
have
shown
that
stretching
skeletal
muscles
during
contraction
(eccentric
contraction)
leads
to
a
long-lasting
muscle
weakness
(Davies
&
White,
1981;
McCully
&
Faulkner,
1985).
Similarly,
stretching
active
single
muscle
fibres
brings
about
a
pronounced
decrease
in
tetanic
force
production
which
persists
for
at
least
1
h
with
no
recovery
(Balnave
&
Allen,
1995).
Part
of
this
force
deficit
was
shown
to
be
the
result
of
a
reduced
intracellular
free
calcium
concentration
([Ca2W]1),
probably
due
to
reduced
Ca2+
release
from
the
sarcoplasmic
reticulum
(SR).
However,
the
maximum
Ca2+-activated
force
was
also
reduced
following
stretch
suggesting
structural
abnormalities
(Balnave
&
Allen,
1995).
Morphological
studies
have
revealed
that
skeletal
muscles
which
have
undergone
eccentric
contractions
in
situ
exhibit
myofibrillar
disorganization
(Armstrong,
Ogilvie
&
Schwane,
1983;
Friden,
Sjostrom
&
Ekblom,
1983;
Wood,
Morgan
&
Proske,
1993).
Commonly
reported
abnormalities
include
sarcomeres
which
appear
to
be
totally
disrupted,
Z-lines
which
have
a
zigzag
appearance,
and
sarcomeres
or
half-
sarcomeres
which
are
overstretched
so
that
there
is
no
overlap
between
myofilaments
(Friden
et
al.
1983;
Wood
et
al.
1993).
The
regions
of
myofibrillar
disorganization
are
often
focal,
with
regions
of
normal
appearance
close
by,
and
are
present
immediately
post-stretch
(Newham,
McPhail,
Mills
&
Edwards,
1983;
Wood
et
al.
1993).
A
single
stretch
during
contraction
is
sufficient
to
generate
this
pattern
of
disorganization
(Brown
&
Hill,
1991;
Brooks,
Zerba
&
Faulkner,
1995;
Talbot
&
Morgan,
1996).
An
equivalent
study
on
the
morphology
of
single
fibres
has
not
been
performed,
so
it
is
unclear
whether
the
stretch-induced
reduction
in
maximum
Ca2P-activated
force
is
due
to
myofibrillar
injury
or
to
some
other
mechanism.
In
the
study
of
Balnave
&
Allen
(1995),
which
showed
that
the
release
of
Ca2+
from
the
SR
was
reduced
following
stretch,
[Ca2+],
was
calculated
from
the
spatially
averaged
fluorescent
Ca2+
signal
obtained
from
approximately
one-third
of
the
muscle
fibre.
Therefore,
these
experiments
could
not
distinguish
between
a
uniform
reduction
in
Ca2+
release
and
a
reduction
at
irregular
intervals
along
the
fibre.
For
instance,
damage
to
T-tubules
might
prevent
inward
conduction
of
the
action
potential
causing
reduced
activation
in
the
centre
of
the
fibre
(Westerblad,
Lee,
Lamb,
Bolsover
&
Allen,
1990;
Duty
&
Allen,
1994).
Alternatively,
there
might
be
a
6441
649

C6
D.
Balnave,
D.
F
Davey
and
D.
0.
Allen
small
number
of
damaged
regions
in
the
fibre
where
C2+
release
was
grossly
reduced.
The
aim
of
the
present
investigation
was
to
determine
the
nature
and
distribution
of
any
sarcomere
disorganization
caused
by
stretching
intact
single
mammalian
skeletal
muscle
fibres
during
contraction.
In
addition,
we
have
studied
the
distribution
of
[Ca2+]1,
both
at
rest
and
during
tetanic
stimulation,
to
determine
whether
the
abnormalities
of
Ca2+
handling
were
uniform
or
showed
some
specific
kind
of
distribution.
The
overall
aim
is
to
explain
the
reduction
in
measured
force
in
terms
of
both
CP
handling
and
sarcomere
organization.
METHODS
Adult,
male
mice
were
killed
by
rapid
cervical
dislocation.
A
single
muscle
fibre
was
dissected
from
the
flexor
brevis
muscle
and
mounted
between
a
force
transducer
and
the
arm
of
a
motor
designed
to
impose
known
length
changes
on
the
fibre.
Details
of
these
procedures
have
been
described
previously
(Balnave
&
Allen,
1995).
Fibres
were
stimulated
with
a
series
of
ten
100
Hz
tetani,
350
ms
in
duration
with
a
4
s
interval
between
each
tetanus.
In
this
preparation
a
100
Hz
tetanus
produces
about
90
%
of
the
maximum
force
obtained
by
raising
the
tetanic
[Ca2+]i
above
maximal
levels
with
caffeine
(Balnave
&
Allen,
1995,
1996).
The
optimum
force-
generating
length
(Lo,
-800
/sm)
was
determined
by
increasing
the
length
of
the
muscle
fibre
from
being
slack
until
tetanic
force
was
maximal.
The
resting
length
of
all
fibres
(stretched
and
control)
was
set
at
100
/um
longer
than
L
so as
to
place
the
fibres
on
the
descending
limb
of
the
force-length
curve.
Fibres
were
stretched
by
either
25
or
50%
L
at
5
muscle
lengths
per
second,
starting
200
ms
after
the
start
of
each
tetanus.
Muscle
length
was
returned
to
its
resting
level
after
completion
of
the
tetanic
stimulation.
For
representative
force
records
see
Balnave
&
Allen
(1995).
Recovery
of
force
was
measured
after
30
min.
In
experiments
requiring
electron
or
confocal
microscopy,
fibres
were
transferred
from
the
experimental
chamber
to
a
second
chamber
designed
for
the
fixation
procedure.
Fibre
length
was
reset
at
approximately
the
same
length
as
in
the
experimental
chamber.
Electron
microscopy
One
unstimulated
fibre
and
one
fibre
which
had
been
stretched
by
50
%
Lo
during
ten
contractions
were
fixed
and
their
fine
structure
examined
using
electron
microscopy.
The
fixative
used
for
electron
microscopy
was
bathing
solution
containing
2
%
glutaraldehyde
and
4%
acrolein
(v/v).
The
fibre
was
fixed
in
place
in
the
experimental
bath.
The
mixture
was
exchanged
for
more
fixative
as
rapidly
as
possible,
but
without
draining
the
solution
below
the
level
of
the
fibre.
After
1
h,
the
fibre
was
cut
from
the
clamps
holding
it
in
the
experimental
apparatus,
transferred
to
a
glass
vial,
and
rinsed
in
several
changes
of
phosphate
buffer
solution
(28
mm
NaH2PO4-
72
mm
Na2HPO4,
pH
7
2).
It
was
fixed
overnight
in
1
%
OSO4
in
the
same
buffer,
and
then
rinsed
with
several
changes
of
buffer
solution
over
a
I
h
period.
It
was
then
dehydrated
through
a
graded
series
of
ethanol
solutions
before
embedding
in
Spurr's
resin
in
an
embedding
capsule.
The
fibre
was
sectioned
at
approximately
50
nm
thickness,
and
stained
with
uranium
and
lead.
Electron
micrographs
were
obtained
with
a
Philips
201c
instrument
at
a
magnification
of
x
6000.
Confocal
microscopy
The
sarcomere
distributions
of
five
fibres
which
had
been
stretched
by
50%
L.,
and
two
fibres
stretched
by
25%
Lo,
during
ten
contractions
were
examined
using
confocal
microscopy.
The
fixative
used
to
prepare
the
muscle
fibres
for
confocal
microscopy
was
4%
paraformaldehyde
in
phosphate
buffer
solution
(28
mm
NaH2PO4
and
72
mm
Na2HPO4).
Once
fixed
a
muscle
fibre
was
placed
in
an
Eppendorf
tube
containing
four
units
of
the
fluorescent
F-actin
stain
rhodamine-phalloidin,
which
had
been
reconstituted
in
200
1
of
a
solution
containing
0-1
M
phosphate
buffer
with
0
5%
Triton
X-100.
The
fibre
was
left
in
the
stain
for
2
days,
before
being
placed
on
a
glass
coverslip
in
the
0-1
M
phosphate
buffer
solution
to
be
imaged
using
confocal
microscopy.
An
inverted
Leica
4D
laser
scanning
confocal
microscope,
with
an
Ar-Kr
laser,
was
used
to
construct
two-dimensional
images
of
the
distribution
of
F-actin
throughout
the
fibres.
The
sample
was
excited
by
light
of
wavelength
568
nm
and
the
emitted
signal
filtered
by
a
590
nm
long-pass
filter.
A
x
40
oil
immersion
objective
lens
with
a
numerical
aperture
of
1-0
was
used
to
scan
50
#im
x
50
,um
sections
of
each
fibre
at
progressively
increasing
depths
of
3
jum
and
representative
images
were
then
stored.
Each
50
um
x
50
jim
section
shared
its
border
with
the
adjoining
section
so
that
the
entire
length
of
each
fibre
was
examined.
The
sarcomere
distributions
of
the
five
fibres
stretched
by
50%
L.
during
ten
contractions
were
compared
with
those
of
seven
control
fibres.
Four
control
fibres
were
not
stimulated,
although
one
of
these
fibres
was
passively
stretched
by
50%
Lo.
The
remaining
three
control
fibres
performed
ten
isometric
contractions.
Calcium
imaging
The
Cai+
was
imaged
along
the
length
of
six
muscle
fibres
which
had
been
stretched
by
50
%
L.
during
ten
contractions.
The
methods
and
equipment
used
for
imaging
Ca2+
in
single
muscle
fibres
have
been
described
previously
(Westerblad
et
al.
1990;
Duty
&
Allen,
1994).
Briefly,
fibres
were
microinjected
with
the
fluorescent
Ca2+
indicator
fura-2.
After
allowing
45
min
for
the
dye
concentration
to
equilibrate
along
the
cell,
the
fibre
was
illuminated
with
ultraviolet
light
of
wavelength
340
or
380
nm
using
an
automated
Nikon
filter
switcher.
An
image
of
the
emitted
fluorescent
light
of
wavelengths
longer
than
430
nm
was
then
obtained.
The
ratio
of
the
image
produced
by
340
nm
illumination
and
the
image
produced
by
380
nm
illumination
could
then
be
converted
to
[Ca!+],
using
the
calibration
procedure
described
by
Westerblad
&
Allen
(1991).
To
obtain
a
ratio
image
of
a
fibre
during
contraction,
images
were
taken
during
two
consecutive
tetani
14s
apart.
The
fibre
was
illuminated
at
340
nm
during
the
first
tetanus
and
at
380
nm
during
the
second
tetanus.
Each
image
was
obtained
by
averaging
over
80
ms,
beginning
200
ms
after
the
start
of
each
tetanus.
Ratio
images
produced
in
this
way
were
taken
at
rest
and
during
100
Hz
tetani
before
and
10,
30
and
60
min
after
the
fibres
were
stretched.
Although
only
about
one-third
of
each
fibre
could
be
examined
in
each
image,
the
pattern
of
the
change
in
[Ca!+]i
was
found
to
be
similar
in
both
the
middle
and
at
the
ends
of
the
fibre.
Statistics
Unless
otherwise
stated
data
are
quoted
as
means
+
S.E.M.
Student's
paired
t
test
was
used
to
verify
statistical
significance
with
P
<
005
taken
as
significant.
650
J
Phy8iol.
502.3

Sarcomere
length
after
stretch
Figure
1.
Electron
micrograph
of
a
longitudinal
section
through
a
control
fibre
and
a
fibre
stretched
by
50
%
Lo
during
contraction
The
unstimulated
control
fibre
(A)
exhibits
a
normal
striation
pattern,
while
the
fibre
which
has
been
stretched
during
contraction
(B)
contains
sarcomeres
which
are
disorganized
in
addition
to
sarcomeres
of
normal
appearance.
The
force
generated
by
100
Hz
stimulation
(100
Hz
force)
following
stretch
was
reduced
to
41
%
of
the
pre-stretch
value.
Scale
bars
represent
2
,im.
J
Physiol.
502.3
651

C.
D.
Balnave,
D.
F
Davey
and
D.
G.
Allen
Figure
2.
For
legend
see
facing
page.
652
J
Physiol.
502.3

Sarcomere
length
after
stretch
RESULTS
Muscle
fibres
stretched
by
50%
L.
during
ten
contractions
showed
significant
reductions
in
tetanic
force.
In
the
twelve
fibres
stretched
by
50%
Lo,
force
generated
by
100
Hz
stimulation
(here
termed
100
Hz
force)
was
reduced
to
36
+
4%
of
the
pre-stretch
force
after
30
min
of
recovery.
In
contrast
in
three
fibres
stimulated
with
ten
isometric
contractions
and
one
fibre
stretched
by
50%
L.
in
the
absence
of
contraction
the
tetanic
force
was
99
8
+
2
3
%
of
the
pre-stretch
force
after
30
min
of
recovery.
These
results
are
similar
to
our
earlier
results
using
the
same
protocol
(Balnave
&
Allen,
1995).
Electron
microscopy
Electron
micrographs
were
taken
of
an
unstimulated
control
fibre
and
a
fibre
which
had
been
stretched
by
50
%
L.
during
ten
contractions
(Fig.
1).
The
control
fibre
in
Fig.
IA
contains
sarcomeres
of
normal
appearance
organized
in
a
regular
array
and
aligned
with
the
sarcomeres
of
neighbouring
myofibrils.
There
is
no
evidence
of
sarcomere
disorganization.
In
contrast,
the
stretched
fibre
in
Fig.
1B
exhibits
many
myofibrillar
abnormalities.
Most
notable
are
Z-lines
which
have
a
wavy
or
zigzag
appearance,
originally
termed
Z-line
streaming
(Friden
et
al.
1983).
In
some
areas
the
Z-lines
are
totally
disrupted.
Consequently,
many
sarcomeres
are
out
of
alignment
with
their
neighbours
and
appear
either
overstretched
or
reduced
in
length.
In
some
regions
the
reduced
overlap
between
myofilaments
is
limited
to
the
half-
sarcomere.
Adjacent
to
these
disorganized
areas
are
regions
of
normal
appearance.
This
pattern
of
injury
has
previously
been
described
in
human
and
whole
muscle
experiments
during
and
immediately
after
the
performance
of
eccentric
muscle
contractions
(Newham
et
al.
1983;
Brown
&
Hill,
1991;
Wood
et
al.
1993;
Brooks
et
al.
1995;
Talbot
&
Morgan,
1996).
Confocal
microscopy
Electron
micrographs
provide
high
resolution
images
but
it
is
difficult
to
scan
spatially
the
fibre
length
with
this
technique.
In
contrast,
with
confocal
microscopy
it
is
possible
to
examine
systematically
sarcomere
length
distribution
throughout
a
fibre.
Figure
2A
shows
an
image
taken
from
an
unstimulated
control
fibre.
Each
bright
band
represents
the
rhodamine-phalloidin-stained
F-actin,
while
each
dark
band
represents
the
H-zone
of
the
sarcomere,
i.e.
the
region
of
the
A-band
where
there
is
no
myofilament
overlap.
Note
that
the
fluorescence
intensity
varies
along
the
bright
band.
The
non-uniform
binding
of
rhodamine-phalloidin
to
actin
filaments
and
the
Z-line
has
been
described
in
skeletal
muscle
myofibrils
by
other
investigators
(Bukatina,
Sonkin,
Alievskaya
&
Yashin,
1984;
Szczesna
&
Lehrer,
1993;
Ao
&
Lehrer,
1995).
In
addition
to
three
unstimulated
control
fibres,
three
control
fibres
were
stimulated
to
produce
ten
contractions
and
another
fibre
was
stretched
by
50%
L.
ten
times
while
at
rest.
As
noted
above,
these
procedures
did
not
affect
the
developed
force.
Each
fibre
was
carefully
scanned
along
its
length
and
at
3
#tm
depths.
All
displayed
a
similar
uniform
appearance
to
the
example
in
Fig.
2A:
sarcomere
length
was
consistent,
the
dark
and
bright
bands
ran
parallel
to
each
other,
and
the
distinction
between
dark
and
bright
bands
was
clear.
In
some
images
we
observed
darker
lines
running
longitudinally
and
parallel
to
the
axis
of
the
fibre
(e.g.
Fig.
2B).
Adjacent
lines
were
spaced
approximately
1
,sm
apart
and
so
may
indicate
the
border
between
neigh-
bouring
myofibrils.
Two
fibres
were
stained
after
being
stretched
by
25%
Lo
during
ten
contractions.
After
30
min
rest
tetanic
force
had
recovered
to
100
and
94
%
of
the
pre-stretch
force
of
each
fibre.
Figure
2B
shows
a
typical
optical
section
of
one
of
these
fibres.
No
sarcomere
inhomogeneities
were
observed
in
any
section
from
either
fibre.
The
confocal
microscope
was
used
to
examine
five
fibres
which
had
been
stretched
by
50
%
Lo
during
ten
contractions
and
stained
with
rhodamine-phalloidin.
All
five
fibres
stretched
by
50
%
Lo
during
contraction
exhibited
sarcomere
length
inhomogeneities
which
were
distributed
throughout
each
fibre.
Confocal
images
of
irregularities
in
the
sarcomere
pattern,
which
may
contribute
to
the
force
deficit,
are
shown
in
optical
sections
from
three
different
fibres
in
Fig.
2C,
D
and
E.
Figure
2C
shows
an
optical
section
of
a
region
in
which
the
sarcomere
spacing
is
clearly
not
uniform.
The
most
obvious
abnormal
region
where
four
sarcomeres
appear
to
be
overextended
is
labelled
with
an
asterisk.
Additionally,
a
smaller
area
of
sarcomere
irregularity,
which
is
more
common,
can
be
observed
at
the
region
labelled
with
Figure
2.
Confocal
images
showing
the
fluorescence
distribution
of
rhodamine-phalloidin
in
a
control
fibre
and
fibres
which
had
been
stretched
during
contraction
A,
confocal
image
of
an
unstimulated
control
fibre.
Note
the
pattern
of
regularly
spaced
bright
bands
indicating
F-actin
fluorescently
stained
with
rhodamine-phalloidin.
B,
fibre
which
had
been
stretched
by
25%
Lo
during
ten
contractions.
The
100
Hz
force
was
reduced
to
94%
of
the
pre-stretch
value
following
30
min
recovery.
Again
note
that
sarcomere
spacing
is
regular
and
uniform.
C-E,
fibres
stretched
by
50%
Lo
during
ten
contractions.
The
100
Hz
force
produced
by
these
fibres
was
reduced
to
50
(C),
30
(D)
and
60%
(E)
of
the
pre-stretch
value
30
min
post-stretch.
C
shows
an
area
with
extremely
overstretched
sarcomeres
(*)
and
a
smaller
area
with
more
focal
sarcomere
inhomogeneity
(t).
D
shows
an
example
of
the
numerous
focal
regions
of
sarcomere
inhomogeneity
located
randomly
throughout
the
fibre.
E
shows
the
zigzag
appearance
of
the
striation
pattern.
Scale
bars
represent
5
#um.
J.
Physiol.
502.3
653

C.
D.
Balnave,
D.
F
Davey
and
D.
C.
Allen
a
dagger.
These
damaged
regions
are
focal
and
do
not
extend
throughout
the
depth
of
the
fibre.
In
fact,
with
the
focal
plane
6
,um
deeper
into
the
fibre
the
sarcomere
pattern
in
this
region
was
essentially
normal.
Therefore,
the
sarcomere
abnormalities
observed
in
Fig.
2C
are
spatially
localized
in
the
z
as
well
as
the
x-y
plane.
A
Control
..
0
.....
OF
.....
.....
.....
Objective
lens
C
PAt,
,I.%
AMIMAf
41
4%
A
1L
M
Aw"*."
V-"WA
I1
im
The
overextended
sarcomeres
shown
in
Fig.
2C
span
the
complete
diameter
of
the
fibre.
However,
more
commonly,
areas
of
sarcomere
inhomogeneity
are
smaller
and
appear
randomly
distributed
within
a
confocal
image
(Fig.
2D).
Another
feature
of
the
fibres
which
had
been
stretched
by
50
%
L.
during
contraction
was
that
in
some
regions
B
Stretch
...
...
Objective
lens
D
Edge
of
fibre
I
Ot
-
A
AA
Middle
of
fibre
it,
1
12
2sm
Edge
of
fibre
4NO44
;
hq
0
300
600
Distance
along
fibre
(,um)
900
0
300
600
Distance
along
fibre
(Cam)
Figure
3.
Variation
in
sarcomere
length
along
an
unstimulated
control
fibre
and
a
fibre
which
had
been
stretched
by
50
%
Lo
during
ten
contractions
Schematic
diagrams
of
cross-sections
through
the
control
fibre
(A)
and
the
stretched
fibre
(B)
(not
drawn
to
scale).
Fibres
have
a
diameter
of
approximately
40
Ism.
The
100
Hz
force
of
the
stretched
fibre
was
reduced
to
30%
of
the
pre-stretch
value
after
30
min
recovery.
Confocal
images
of
the
entire
length
of
each
fibre
were
taken
at
five
equally
spaced
depths
(approximately
10
jcum),
beginning
and
ending
at
the
upper
and
lower
surfaces
of
the
fibre,
respectively.
At
each
depth,
sarcomere
spacing
was
measured
at
five
equally
spaced
intervals
(approximately
10
/um)
across
the
fibre,
again
beginning
and
ending
at
the outer
edges
of
the
fibre.
Each
circle
and
bar
represents
the
mean
+
S.D.
of
the
length
of
all
the
sarcomeres
measured
along
the
fibre
in
the
zone
indicated
by
the
position
of
the
circle
relative
to
the
mean
fibre
sarcomere
length
(3-26
,um
for
A,
3X1
#m
for
B)
represented
by
the
dashed
line
at
each
level.
The
standard
deviations
are
much
larger
in
the
stretched
fibre.
C
and
D
show
individual
records
of
the
variation
in
sarcomere
length
along
the
control
fibre
(C)
and
the
stretched
fibre
(D)
at
the
positions
marked
by
the
open
circles
in
A
and
B,
respectively.
Note
the
much
larger
variation
in
sarcomere
length
along
the
stretched
fibre.
900
654
J
Physiol.
502.3
r
h
sarcomeres
appeared
out
of
alignment
with
their
neighbours.
This
occurred,
in
particular,
at
the
longitudinally
orientated
lines
which
may
represent
the
border
between
adjacent
myofibrils
(Fig.
2E).
Therefore,
in
addition
to
sarcomere
length
inhomogeneities,
this
gave
the
striation
pattern
a
zigzag
appearance.
Histogram
of
sarcomere
length
In
all
five
fibres
stretched
by
50%
Lo
during
contraction
sarcomere
disturbances
were
distributed
randomly
through-
out
the
fibre.
A
detailed
analysis
of
the
sarcomere
spacing
from
one
of
the
five
fibres
that
had
been
stretched
by
50
%
Lo
during
contraction
and
one
unstimulated
control
fibre
is
shown
in
Fig.
3.
Sarcomere
length
was
calculated
as
the
distance
between
the
centres
of
consecutive
bright
bands
on
a
confocal
image.
In
the
majority
of
instances
the
centre
of
the
bright
band,
which
denotes
a
Z-line,
was
marked
by
a
distinct
peak
in
fluorescence
intensity.
Figure
3A
shows
a
schematic
diagram
of
a
cross-section
through
the
control
fibre.
Fibres
have
a
diameter
of
approximately
40
,um.
The
length
of
every
sarcomere
along
the
fibre
was
measured
at
a
depth
and
breadth
indicated
by
the
position
of
the
circles.
Each
circle
represents
the
mean
sarcomere
length
of
all
the
sarcomeres
along
the
fibre
in
that
655
zone.
Thus,
Fig.
3A
illustrates
the
extent
to
which
the
mean
sarcomere
length
of
each
zone
fluctuated
from
the
mean
sarcomere
length
of
the
whole
fibre
(dashed
lines)
and
the
degree
to
which
sarcomere
length varied
in
each
zone
(bars
indicate
+
1
standard
deviation
(S.D.))
for
the
control
fibre.
The
equivalent
measurements
in
the
stretched
fibre
are
shown
in
Fig.
3B.
Note
that,
although
the
mean
sarcomere
length
of
each
zone
did
not
deviate
greatly
from
the
mean
sarcomere
length
of
the
whole
fibre
in
either
cell,
individual
sarcomere
lengths
were
significantly
more
variable
(P
<
0-001;
Levene
median
test
for
equal
variance)
following
stretch
than
in
the
control
fibre.
This
variability
in
sarcomere
length
following
stretch
was
observed
in
each
zone
analysed.
Individual
records
of
this
sarcomere
length
distribution
(taken
from
the
zones
indicated
by
open
circles
in
Fig.
3A
and
B)
are
shown
respectively
for
the
control
and
stretched
fibres
in
Fig.
3C
and
D.
The
greater
variability
in
sarcomere
length
following
stretch
compared
with
the
control
fibre
is
apparent.
This
variability
can
be
observed
along
the
entire
length
of
the
fibre
(Fig.
3D).
Occasionally
there
are
spikes
corresponding
to
highly
overstretched
or
supercontracted
sarcomeres.
Note
that
the
overstretched
sarcomeres
are
not
necessarily
found
immediately
next
to
the
very
short
sarcomeres.
A
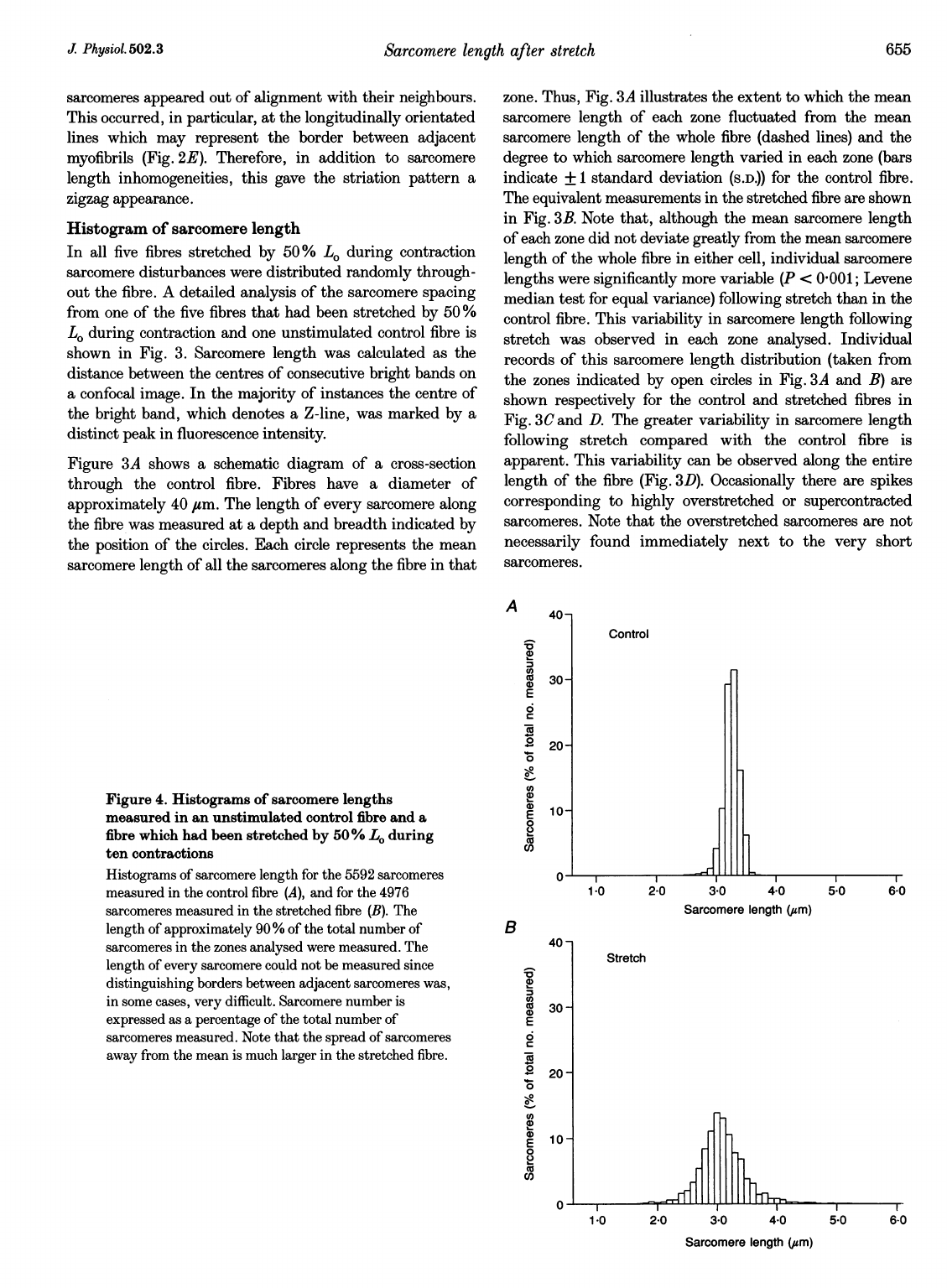
Figure
4.
Histograms
of
sarcomere
lengths
measured
in
an
unstimulated
control
fibre
and
a
fibre
which
had
been
stretched
by
50%
L.
during
ten
contractions
Histograms
of
sarcomere
length
for
the
5592
sarcomeres
measured
in
the
control
fibre
(A),
and
for
the
4976
sarcomeres
measured
in
the
stretched
fibre
(B).
The
length
of
approximately
90
%
of
the
total
number
of
sarcomeres
in
the
zones
analysed
were
measured.
The
length
of
every
sarcomere
could
not
be
measured
since
distinguishing
borders
between
adjacent
sarcomeres
was,
in
some
cases,
very
difficult.
Sarcomere
number
is
expressed
as
a
percentage
of
the
total
number
of
sarcomeres
measured.
Note
that
the
spread
of
sarcomeres
away
from
the
mean
is
much
larger
in
the
stretched
fibre.
')
co
co
0)
-a
2
0
0
o
(D
E
0
2
o
eU
B
CD
CU
co
0
.2
0
0
e?
CD
11
Control
1-0
2-0 3-0
4-0
Sarcomere
length
(usm)
40-
30
-
20-
10-
5-0
6-0
Stretch
v
,I
1.0
2-0
3-0
4-0
5-0
6-0
Sarcomere
length
(,am)
J
Physiol.502.3
Sarcomere
length
after
stretch
_,,.rr

C.
D.
Balnave,
D.
F
D
A
histogram
incorporating
the
length
of
every
sarcomere
measured
from
the
confocal
images
is
shown
in
Fig.
4.
Figure
4A
shows
the
histogram
of
sarcomere
lengths
in
the
control
fibre.
A
total
of
5592
sarcomeres
were
measured.
The
mean
sarcomere
length
was
3-26
ium,
with
the
majority
of
sarcomeres
(>60%)
between
3-2
and
3-3,um.
This
equated
to
a
standard
deviation
of
0
14
gum.
Since
the
fibre
was
fixed
at
a
length
of
100
gm
longer
than
L.,
the
optimum
sarcomere
length
is
estimated
as
2-86
gm.
This
value
compares
with
the
values
reported
by
other
investigators
who
measured
optimum
sarcomere
lengths
in
mammalian
skeletal
muscle
fibres
of
-2
8
gum
(Rack
&
Westbury,
1969;
Stephenson
&
Williams,
1982;
Balnave
&
Allen,
1996).
Similar
results
were
obtained
from
a
fibre
which
had
been
stimulated
to
produce
ten
isometric
contractions
(537
sarcomeres
measured;
S.D.,
0
09
gum)
and
in
the
two
fibres
stretched
by
25
%
L.
during
contraction
(497
and
557
sarcomeres
measured;
S.D.,
0'07
and
0
09
gim,
respectively).
To
construct
the
histogram
of
sarcomere
lengths
in
the
fibre
stretched
by
50%
L.
during
contraction
(Fig.
4B)
4976
sarcomeres
were
measured.
The
mean
sarcomere
length
in
this
fibre
was
3-10,um,
which
corresponds
to
an
optimum
sarcomere
length
of
2-67
gim.
However,
in
contrast
to
the
control
fibre,
60
%
of
sarcomeres
had
lengths
spread
between
2-8
and
3-3
gim,
which
equated
to
a
standard
Resting
100
Hz
tetanus
10
min
Pre-stretch
post-stretch
pc
100i
xm
avey
and
D.
0.
Allen
J
Physiol.
502.3
deviation
of
0
40
gm.
Therefore,
the
sarcomere
lengths
in
the
stretched
fibre
were
far
more
variable
compared
with
the
control
fibre,
but
the
distribution
of
variability
shows
no
obvious
pattern.
Imaging
Ca2+
release
We
have
previously
shown
that
the
tetanic
[CaP+]i
is
reduced
following
stretch-induced
injury
(Balnave
&
Allen,
1995).
However,
these
studies
give
no
indication
of
the
distribution
of
this
reduction
in
[Ca2P]1.
For
instance,
T-tubular
damage
might
lead
to
radial
gradients
of
[Ca2+],
(Westerblad
et
al.
1990;
Duty
&
Allen,
1994).
Therefore,
using
the
fluorescent
Ca2+
indicator
fura-2,
we
imaged
[Ca2+]i
in
fibres
stretched
by
50
%
L.
during
contraction.
Figure
5
shows
images
of
the
middle
third
of
a
typical
fibre
taken
at
rest
and
during
a
100
Hz
tetanus
before
and
10,
30
and
60
min
post-stretch.
At
rest
(blue)
[Ca2P]i
was
slightly
higher
after
stretch,
as
indicated
by
the
lighter
shade
of
blue
in
the
images.
However,
with
the
resolution
of
this
imaging
system,
there
was
no
evidence
of
an
uneven
distribution
of
[Ca2+]i
within
the
resting
fibre,
nor
was
the
standard
deviation
of
the
[Ca2P]i
in
all
pixels
changed.
This
observation
was
consistent
in
the
six
fibres
analysed.
Therefore,
it
seems
unlikely
that
stretching
a
contracting
muscle
fibre
causes
the
surface
membrane
to
tear
since
we
30
min
60
min
)st-stretch
post-stretch
nfl
Ratio
19
2
0
Figure
5.
Pseudocolour
ratio
images
from
the
middle
third
of
one
fibre
stretched
by
50%
Lo
during
contraction
showing
the
distribution
of
[Ca2+]j
along
the
fibre
at
rest
and
during
a
100
Hz
tetanus
Images
were
taken
prior
to
stretch
and
10,
30
and
60
min
post-stretch.
In
this
fibre
100
Hz
force
was
reduced
to
31
%
following
60
min
recovery.
The
colours
on
the
calibration
bar
indicate
the
fluorescence
ratio
of
fura-2.
The
relationship
between
fura-2
ratio
and
[Ca?+],
is
approximately:
0
7
_
70
nM,
10
0465
nM,
1-2
_
1200
nM.
656
1

Sarcomere
length
after
stretch
observed
no
localized
regions
with
a
high
resting
[Ca2+]i
where
a
damaged
section
of
surface
membrane
should
allow
Ca
to
enter
the
fibre
along
its
large
concentration
gradient.
Similarly,
in
the
fibre
displayed
in
Fig.
5,
the
distribution
of
[Ca2+]i
during
a
100
Hz
tetanus
was
uniform
in
the
hour
after
stretch.
The
paler
yellow
colour
post-stretch
indicates
that
tetanic
[Ca2P]i
is
reduced.
In
the
six
fibres
analysed
tetanic
[Ca2+]
was
reduced
from
664
+
68
to
501
+
30
nm
(P
<
0
05)
after
1
h
recovery.
However,
there
were
no
detectable
longitudinal
or
radial
gradients
of
[Ca2+],
and
the
standard
deviation
of
the
[Ca2P]i
in
all
pixels
was
smaller
following
stretch.
DISCUSSION
Stretching
intact
single
mammalian
skeletal
muscle
fibres
during
contraction
has
been
shown
to
bring
about
a
reduction
of
tetanic
force
which
lasts
for
at
least
1
h.
In
a
previous
investigation,
the
results
of
which
have
been
confirmed
in
the
present
study,
we
showed
that
this
stretching
protocol
resulted
in
a
reduced
tetanic
[Ca2P]1
(Balnave
&
Allen,
1995).
This
provided
more
direct
evidence
for
an
earlier
suggestion
that
stretch
during
contraction
can
cause
reduced
Ca2+
release
from
the
SR
(Warren,
Lowe,
Hayes,
Karwoski,
Prior
&
Armstrong,
1993).
In
our
earlier
study
we
showed
that
stretching
muscle
fibres
by
25%
L.
produced
a
force
deficit
which
could
be
completely
accounted
for
by
the
reduced
SR
Ca2P
release.
However,
when
the
severity
of
the
stretching
protocol
was
increased,
by
stretching
the
muscle
fibres
by
50%
Lo,
we
observed
an
additional
reduction
in
the
maximum
Ca2+-activated
force
which
we
attributed
to
sarcomere
disorganization,
although
no
structural
evidence
for
this
was
presented
(Balnave
&
Allen,
1995).
Electron
microscopy
The
electron
micrographs
of
the
stretched
fibre
revealed
that
abnormalities
in
the
sarcomere
pattern
are
quantitatively
similar
to
those
described
in
human,
animal
and
whole
muscle
experiments
by
other
investigators
(Armstrong
et
al.
1983;
Friden
et
at.
1983;
Newham
et
al.
1983;
Wood
et
al.
1993).
Therefore,
the
single
fibre
model
of
stretch-induced
muscle
injury
is
analogous
to
the
whole
animal
condition
structurally
as
well
as
functionally
(Balnave
&
Allen,
1995).
Because
it
is
very
difficult
to
sample
systematically
along
a
fibre
using
electron
microscopy,
we
used
confocal
microscopy
to
obtain
a
description
of
the
sarcomere
length
disruption
throughout
a
single
fibre.
Confocal
microscopy
In
a
previous
investigation
neither
ten
isometric
contractions
nor
ten
stretches
of
50
%
Lo
in
resting
fibres
produced
a
force
deficit
(Balnave
&
Allen,
1995).
Stretching
muscle
fibres
by
25%
Lo
during
ten
contractions
was
shown
to
reduce
tetanic
Ca2+
release
but
did
not
affect
the
maximum
CaP-activated
force.
In
the
present
study
no
notable
sarcomere
length
inhomogeneity
was
observed
in
any
of
the
(ii)
unstimulated
fibres
stretched
ten
times
by
50%
L.,
(iii)
fibres
stimulated
with
ten
isometric
contractions,
and
(iv)
fibres
stretched
by
25%
L.
during
ten
contractions.
However,
in
all
five
fibres
which
had
performed
stretches
of
50
%
L.
during
contraction,
multiple
areas
of
sarcomere
length
inhomogeneity
of
varying
degrees
were
observed
using
confocal
microscopy.
Therefore,
it
appears
likely
that
the
reduction
in
the
maximum
Ca2P-activated
force,
observed
after
stretching
a
contracting
muscle
fibre
by
50
%
L1
(Balnave
&
Allen,
1995),
is
the
result
of
stretch-induced
sarcomere
disorganization.
We
have
shown
that
stretching
a
muscle
fibre
by
50%
L1
during
ten
contractions
causes
force
to
fall
to
36
+
4%
and
produces
severe
sarcomere
disruption.
However,
a
25
%
stretch
produced
no
force
deficit
or
sarcomere
inhomogeneity.
The
50%
stretch
is
very
large
and
it
can
be
questioned
whether
this
result
is
relevant
to
events
which
occur
in
intact
muscles.
Although
a
50%
stretch
is
large
it
is
still
within
the
range
which
can
occur
in
muscles
(Brooks
et
al.
1995)
and
the
reduction
in
force
which
we
observe
is
similar
to
that
reported
by
others
in
the
literature.
For
instance,
Brooks
et
al.
(1995)
found
that
a
single
stretch
of
less
than
30
%
produced
no
reduction
in
force,
while
a
single
stretch
of
60%
reduced
force
to
35%.
These
results
from
intact
in
situ
muscles
are
not
greatly
different
from
ours
in
isolated
single
fibres.
Histogram
of
sarcomere
length
The
sarcomere
length
inhomogeneity
can
be
distinguished
clearly
by
examining
the
histograms
of
sarcomere
length
from
the
control
and
stretched
fibres.
Sarcomere
length
in
the
control
fibre
ranged
from
2-3
,sm
(mainly
at
the
ends
of
the
fibre
where
sarcomere
length
was
shorter
than
the
mean
value;
Fig.
3C)
to
3-7
,am.
In
contrast,
sarcomere
length
in
the
stretched
fibre
ranged
from
1'7
to
5-9
/sm.
This
variability
is
reflected
in
the
standard
deviations
of
sarcomere
length
of
0-14
and
0
40,um
for
the
control
and
stretched
fibres,
respectively.
Sarcomere
inhomogeneities
following
contractions
with
stretch
have
been
recognized
for
many
years
(e.g.
Newham
et
al.
1983).
Morgan
(1990)
developed
and
quantified
these
ideas
and
proposed
the
'popping
sarcomere'
hypothesis
to
explain
many
features
of
contractions
with
stretch.
Morgan
suggested
that
when
a
stretch
is
imposed
on
a
contracting
muscle
the
lengthening
of
individual
sarcomeres
is
not
uniform.
Due
to
biological
variation
some
sarcomeres
will
be
weaker
than
others.
The
weakest
sarcomere
tends
to
stretch
the
most
and
once
the
sarcomere
reaches
the
point
on
its
force-velocity
curve
when
velocity
of
stretch
increases
independently
of
force
it
elongates
extremely
rapidly
and
uncontrollably
(popping).
If
muscle
fibre
lengthening
continues
after
the
weakest
sarcomere
has
popped
then
the
next
weakest
sarcomere
will
elongate,
and
so
on
until
the
stretch
is
complete.
Upon
relaxation
it
was
proposed
that
some
of
the
extended
sarcomeres
do
not
return
to
the
following
conditions;
(i)
unstimulated,
unstretched
fibres,
J
Physiol.
502.3
657
interdigitating
pattern
but
remain
overextended.

C.
D.
Balnave,
D.
F
Davey
and
D.
C.
Allen
In
his
popping
sarcomere
hypothesis
Morgan
(1990)
suggested
that
the
weakest
sarcomeres
are
randomly
distributed
throughout
a
muscle
fibre.
Our
results
support
this
idea
of
a
random
distribution
of
overstretched
sarcomeres.
However,
we
also
observed
many
sarcomeres
of
very
short
length.
Morgan's
theory
predicts
that
muscle
fixed
during
a
single
contraction
with
stretch
should
produce
a
small
peak
in
the
sarcomere
length
histogram
at
a
long
sarcomere
length,
representing
occasional
regions
of
over-
stretched
sarcomeres,
and
a
large
peak
at
a
sarcomere
length
slightly
shorter
than
the
mean
length,
representing
an
evenly
distributed
shortening
of
the
remaining
sarcomeres.
This
prediction
has
subsequently
been
confirmed
(Brown
&
Hill,
1991;
Talbot
&
Morgan,
1996).
Our
results
show
that,
after
recovery
from
ten
contractions
with
stretch,
in
addition
to
overstretched
sarcomeres
the
length
of
some
sarcomeres
is
dramatically
reduced
while
at
least
25%
remain
within
0
5
,um
of
the
mean
sarcomere
length.
Although
the
distribution
of
sarcomeres
is
not
what
would
be
predicted
from
Morganes
hypothesis
this
may
be
because
in
our
experiments
sarcomere
length
was
measured
after
the
fibre
had
relaxed
and
returned
to
the
control
length
before
fixing.
It
is
possible
that
passive
restoring
forces
cause
some
over-
stretched
sarcomeres
to
resume
their
interdigitation
during
and
after
relaxation
and
there
may
also
be
other
processes
leading
to
redistribution
of
sarcomere
lengths.
Imaging
Ca2+
release
Our
results
confirm
earlier
studies
that
have
shown
that
100
Hz
tetanic
[Ca2+]i
is
reduced
following
stretch-induced
injury
(Warren
et
al.
1993;
Balnave
&
Allen,
1995),
but
show
that
the
distribution
of
the
reduced
[Ca2+]i
is
uniform,
at
least
at
the
resolution
of
the
present
imaging
system.
This
result
allows
us
to
exclude
the
possibility
that
T-tubular
damage
leading
to
a
uniform
failure
of
inward
spread
of
activation
occurs,
such
as
that
detected
in
some
types
of
muscle
fatigue
(Westerblad
et
al.
1990;
Duty
&
Allen,
1994).
It
is
also
clear
that
stretch-induced
injury
does
not
lead
to
a
small
number
of
restricted
areas
of
reduced
Ca2+
release,
as
this
would
be
very
obvious
in
the
images.
Another
possibility
is
that
the
multiple
sites
of
sarcomere
disorganization
seen
in
the
electron
micrograph
and
confocal
images
are
each
associated
with
similar
regions
of
reduced
Ca2+
release.
To
try
to
detect
this
kind
of
spatial
variability
of
Ca2+
release
we
compared
the
standard
deviation
of
Ca2+
across
all
pixels.
The
standard
deviation
was
lower
following
stretch,
suggesting
that
spatial
variability
is
not
increased.
However,
the
resolution
of
conventional
imaging
is
reduced
in
thick
specimens
because
of
the
contribution
of
out-of-focus
light
(Sandison
&
Webb,
1994)
and
it
remains
possible
that
a
higher
resolution
method
will
detect
localized
regions
of
reduced
Ca2+
release
which
have
the
same
distribution
as
the
regions
of
sarcomere
damage.
Conclusion
In
conclusion,
stretching
intact
single
mammalian
skeletal
muscle
fibres
during
contraction
leads
to
structural
disorganization
of
the
contractile
apparatus
similar
to
that
observed
in
whole
animal
and
whole
muscle
investigations.
Confocal
microscopy
can
be
used
to
analyse
sarcomere
inhomogeneity
in
these
stretched
fibres
and
shows
that
sarcomere
length
is
extremely
variable
throughout
such
fibres.
Conventional
wide-field
fluorescence
imaging
microscopy
has
been
used
to
show
that
tetanic
and
resting
[Ca2+]i
are
uniformly
distributed
along
these
single
fibres
post-stretch.
This
finding
suggests
that
reduced
Ca2+
release
occurs
regularly
throughout
stretched
muscle
fibres.
Ao,
X.
L.
&
LEHRER,
S.
S.
(1995).
Phalloidin
unzips
nebulin
from
thin
filaments
in
skeletal
myofibrils.
Journal
of
Cell
Science
108,
3397-3403.
ARMSTRONG,
R.
B.,
OGILVIE,
R.
W.
&
SCHWANE,
J.
A.
(1983).
Eccentric
exercise-induced
injury
to
rat
skeletal
muscle.
Journal
of
Applied
Physiology
54,
80-93.
BALNAVE,
C.
D.
&
ALLEN,
D.
G.
(1995).
Intracellular
calcium
and
force
in
single
mouse
muscle
fibres
following
repeated
contractions
with
stretch.
Journal
of
Physiology
488,
25-36.
BALNAVE,
C.
D.
&
ALLEN,
D.
G.
(1996).
The
effect
of
muscle
length
on
intracellular
calcium
and
force
in
single
fibres
from
mouse
skeletal
muscle.
Journal
of
Physiology
492,
705-713.
BROOKS,
S.
V.,
ZERBA,
E.
&
FAULKNER,
J.
A.
(1995).
Injury
to
muscle
fibres
after
single
stretches
of
passive
and
maximally
stimulated
muscles
in
mice.
Journal
of
Physiology
488,
459-469.
BROWN,
L.
M.
&
HILL,
L.
(1991).
Some
observations
on
vAriations
in
filament
overlap
in
tetanized
muscle
fibres
and
fibres
stretched
during
a
tetanus,
detected
in
the
electron
microscope
after
rapid
fixation.
Journal
of
Muscle
Research
and
Cell
Motility
12,
171-182.
BUKATINA,
A.
E.,
SONKIN,
B.
Y.,
ALIEVSKAYA,
L. L.
&
YASHIN,
V.
A.
(1984).
Sarcomere
structures
in
the
rabbit
psoas
muscle
as
revealed
by
fluorescent
analogs
of
phalloidin.
Histochemistry
81,
301-304.
DAvIES,
C.
T.
M.
&
WHITE,
M.
J.
(1981).
Muscle
weakness
following
eccentric
work
in
man.
Pflfigers
Archiv
392,
168-171.
DUTY,
S.
&
ALLEN,
D.
G.
(1994).
The
distribution
of
intracellular
calcium
concentration
in
isolated
single
fibres
of
mouse
skeletal
muscle
during
fatiguing
stimulation.
Pfluigers
Archiv
427,
102-109.
FRIDEN,
J.,
SJOSTROM,
M.
&
EKBLOM,
B.
(1983).
Myofibrillar
damage
following
intense
eccentric
exercise
in
man.
International
Journal
of
Sports
Medicine
4,
170-176.
MCCULLY,
K.
K.
&
FAULKNER,
J.
A.
(1985).
Injury
to
skeletal
muscle
fibers
of
mice
following
lengthening
contractions.
Journal
of
Applied
Physiology
59,
119-126.
MORGAN,
D.
L.
(1990).
New
insights
into
the
behavior
of
muscle
during
active
lengthening.
Biophysical
Journal
57,
209-221.
NEWHAM,
D.
J.,
MCPHAIL,
G.,
MILLS,
K.
R.
&
EDWARDS,
R.
H.
T.
(1983).
Ultrastructural
changes
after
concentric
and
eccentric
contractions
of
human
muscle.
Journal
of
the
Neurological
Sciences
61,
109-122.
RACK,
P.
M.
H.
&
WESTBURY,
D.
R.
(1969).
The
effects
of
length
and
stimulus
rate
on
tension
in
the
isometric
cat
soleus
muscle.
Journal
of
Physiology
204,
443-460.
SANDISON,
D.
R.
&
WEBB,
W.
W.
(1994).
Background
rejection
and
signal-to-noise
optimization
in
confocal
and
alternative
fluorescence
microscopes.
Applied
Optics
33,
603-615.
STEPHENSON,
D. G.
&
WILLIAMS,
D.
A.
(1982).
Effects
of
sarcomere
length
on
the
force-pCa
relation
in
fast-
and
slow-twitch
skinned
muscle
fibres
from
the
rat.
Journal
of
Physiology
333,
637-653.
658
J
Phy8iol.
502.3

Sarcomere
length
after
stretch
SZCZESNA,
D.
&
LEHRER,
S. S.
(1993).
The
binding
of
fluorescent
phallotoxins to
actin
in
myofibrils.
Journal
of
Mu8cle
Research
and
Cell
Motility
14,
594-597.
TALBOT,
J.
A.
&
MORGAN,
D.
L.
(1996).
Quantitative
analysis
of
sarcomere
non-uniformities
in
active
muscle
following
a
stretch.
Journal
of
Muscle
Research
and
Cell
Motility
17,
261-268.
WARREN,
G.
L.,
LOWE,
D.
A.,
HAYES,
D.
A.,
KARWOSKI,
C.
J.,
PRIOR,
B.
M.
&
ARMSTRONG,
R.
B.
(1993).
Excitation
failure
in
eccentric
contraction-induced
injury
of
mouse
soleus
muscle.
Journal
of
Physiology
468,
487-499.
WESTERBLAD,
H.
&
ALLEN,
D.
G.
(1991).
Changes
of
myoplasmic
calcium
concentration
during
fatigue
in
single
mouse
muscle
fibers.
Journal
of
General
Physiology
98,
615-635.
WESTERBLAD,
H.,
LEE,
J.
A.,
LAMB,
A.
G.,
BoLsovER,
S.
R.
&
ALLEN,
D.
G.
(1990).
Spatial
gradients
of
intracellular
calcium
in
skeletal
muscle
during
fatigue.
Pfluigers
Archiv
415,
734-740.
WOOD,
S.
A.,
MORGAN,
D.
L.
&
PROSKE,
U.
(1993).
Effects
of
repeated
eccentric
contractions
on
structure
and
mechanical
properties
of
toad
sartorius
muscle.
American
Journal
of
Physiology
265,
C792-800.
Acknowledgements
This
work
was
supported
by
the
National
Health
and
Medical
Research
Council
of
Australia.
The
authors
would
also
like
to
thank
Dr
Stewart
Head
and
Ms
Ann
Parkinson
for
their
advice
on
the
rhodamine-phalloidin
staining
technique.
Author's
email
address
D.
G.
Allen:
Received
18
December
1996;
accepted
23
April
1997.
J
Physiol.
502.3
659
